More Information
Submitted: August 10, 2024 | Approved: August 16, 2024 | Published: August 19, 2024
How to cite this article: Minaev B. Magnetic Properties of Reactive Oxygen Species and their Possible Role in Cancer Therapy. Arch Cancer Sci Ther. 2024; 8(1): 048-053. Available from: https://dx.doi.org/10.29328/journal.acst.1001044
DOI: 10.29328/journal.acst.1001044
Copyright License: © 2024 Minaev B. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Magnetic Properties of Reactive Oxygen Species and their Possible Role in Cancer Therapy
Boris Minaev*
The Bogdan Khmelnytsky National University, Cherkasy, Ukraine
*Address for Correspondence: Boris Minaev, The Bogdan Khmelnytsky National University, Bvd. Shevchenko 81, Cherkasy 18031, Ukraine, Email: [email protected]
Spin-depending internal magnetic interactions in oxygen are crucial for the chemistry and photobiology of this molecule. Photosynthesis, respiration, and many other life-supporting oxygen reactions are governed by enzymes that use fine magnetic forces to overcome the spin-forbidden character of all aerobic metabolism. Life on Earth occurs on the border between combustion and oxidative phosphorylation, and this balance is largely dependent on reactive oxygen species. ROS can cause apoptosis or cell necrosis, and ROS also controls homeostasis through numerous signaling functions. Until recently, biochemists had not paid attention to internal magnetic interactions that influence the chemical activity of such ROS as superoxide ion, singlet oxygen, peroxynitrite, and many others. The role of superoxide dismutase, the oldest enzyme on the Earth, which provides superoxide concentration control, stresses the importance of the O2-• species as the precursor of many other ROS. Spin-orbit coupling in O2-• and O2 species are the main internal magnetic interactions that could influence cancer growth and be connected with cancer therapy.
Reactive oxygen species (ROS) form a family of highly reactive radicals and neutral compounds that have evolved during aerobic evolution to provide some cellular signaling functions [1]. Most ROS are short-lived derivatives of molecular oxygen temporarily generated as byproducts of metabolic O2 reactions with organic stuff. Such reactions are strictly forbidden by the spin-selection rule of quantum chemistry since the O2 molecule is a triplet biradical with nonzero intrinsic magnetic moment determined by two spin-unpaired electrons, while most organic molecules (amino acids, DNA, RNA, vitamins, etc.) are typical diamagnetics [2,3]. That is, they possess an even number of electrons those spins are all paired and the total spin of each molecule is zero (S=0). Spin is an inalienable property of electrons like mass and electric charge, but it has no analogy in classical physics and was established in quantum electrodynamics [3,4]. Simply speaking, the electron is a tiny magnet induced by spin angular momentum, whose orientation in space is possible in two opposite directions. The spin quantum number of one electron S=1/2, and its projection on the magnetic field axis can be equal to MS = ±ħ/2, where ħ is the Planck constant divided by 2π [5]. The even number of electrons in organic molecules are distributed on the low-lying energy levels of molecular orbitals (MOs) according to the Pauli principle in such a way that each MO can be occupied by two electrons with opposite spins. Thus, the total electronic spin of organic molecules is quenched (S=0); such molecules do not possess an intrinsic magnetic moment. Thus, the organic molecules are repulsed from an external magnetic field (EMF) since EMF induces the electronic orbital rotation inside the molecule which provides a low magnetic moment directed against EMF. This magnetic property is called diamagnetism [5].
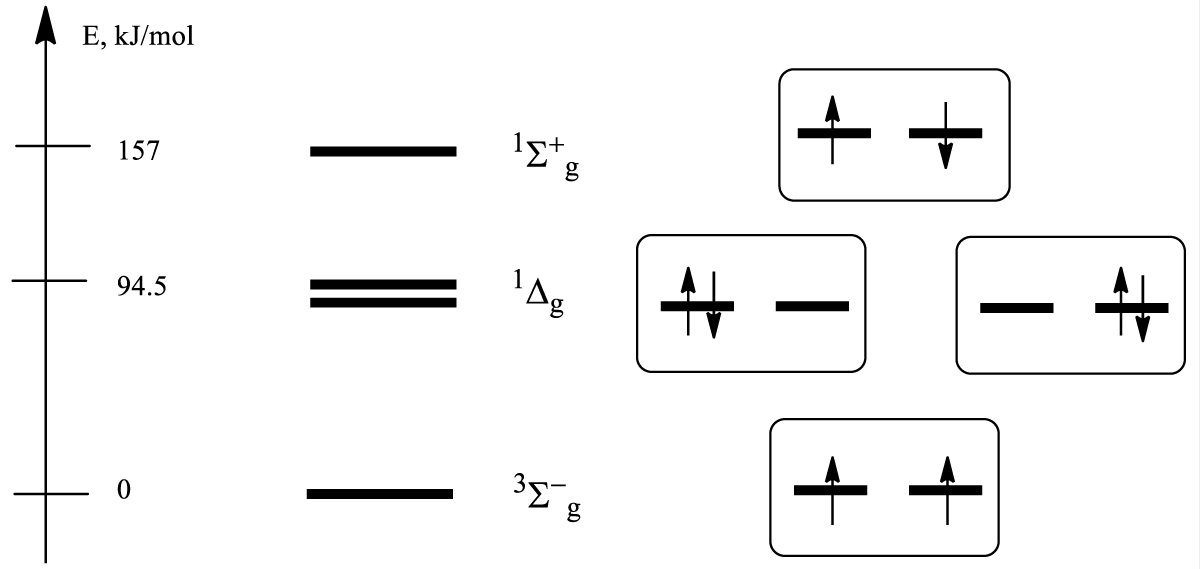
The two highest occupied MOs in the O2 molecule πg,x, and πg,y have the same energy (they are degenerate) because of molecular symmetry (Figure 1). According to the Hund rule, each MO is occupied by one electron and both have the same spin orientation [6]. Thus, their spin moments add up and the total spin is equal to S=1/2+1/2=1 with three possible projections MS = ±ħ, 0 on the molecular axis. This determines the notion of the triplet ground state of the O2 molecule ( , Figure 1) and its intrinsic magnetic moment (μ = eħ/mc), where e, m are the charge and mass of an electron, c – light velocity. According to Faraday’s classification, oxygen is a paramagnetic gas; its molecules are attracted by external magnetic fields and oxygen weight increases on magnetic scales (in contrast to diamagnetic gases CO2, H2O, and N2, whose weight decreases).
Figure 1: Energy levels of low-lying states of the oxygen molecule and their electronic configurations. Only one spin sublevel of the ground triplet state (Ms = +ħ) is shown.
The oxygen molecule provides a background for all aerobic life on the Earth. This is a vital elixir from green plants beneath the Sun. We inhale oxygen to oxidize food and obtain energy in the form of adenine triphosphates (ATP) for life: for protein synthesis, muscle movement, and neuron activation. However, the respiration process in mitochondria (the Krebs cycle of lemon acid)
(1)
is strictly forbidden by the spin-selection rule from the quantum physics perspective [2]. To promote the Krebs cycle process (1) we need to change the spin orientation of a single electron in the left part of Equation (1). The total spin on the left corresponds to the triplet state (S=1), but on the right, it is singlet (S=0) and the process is spin-forbidden. The spin transition from the triplet to the singlet state can be induced only by intrinsic magnetic force, but in organic chemistry such forces are reasonably neglected [2]. Explanation of this puzzle is coded by the term “enzyme”; it contains cytochrome c oxidase with two paramagnetic ions Fe(II) and Cu(II) in the enzyme active center. The 3d atomic orbitals (AO) with five quasi-degenerate sublevels and different angular momentum projections LZ = ±2ħ, ±1ħ 0 easily provide non-paired electron spins in weak ligand fields of enzymes. Oxygen reaction in the coordination sphere of these ions is not formally spin forbidden [4]. The rate constant of such a reaction depends on the exchange interaction (EI) between spins of O2 and paramagnetic metal ions [5,6]. EI has an electronic repulsion nature but depends on spins according to the quantum mechanics of many electron systems governed by the Pauli principle [6].
Oxygen is a good oxidizing reactant from the thermodynamics argument; however, spin prohibition strictly prevents the spontaneous oxidation of organic fuels (A) by combustion in the open air [2]. Products of full combustion (P) are stable diamagnetic substances: carbon dioxide, nitrogen, and water. They cannot be obtained by direct reactions between triplet oxygen and singlet organic molecules: A (↑↓) + O2(↑↑) =x=> P (↑↓)(↑↓) [7]. Thus, they proceed through the radical chain reactions initiated by match: A (↑↓) + O2(↑↑) + R (↓) = P (↑↓)(↑↓) + Rꞌ(↑) [2]. This reaction is spin-allowed. The high temperature of match flame creates a first radical R(↓), a fragment from the decay of molecule A produced by chemical bond cleavage and possessing a non-paired electron spin. The total spin S=1/2 and MS=+1/2 quantum numbers are the same in the left and right parts of this reaction. One can see that the spin exchange between O2(↑↑) and R (↓) makes the deal. This simple mechanistic trick lies in the background of the complicated quantum chemical mechanism of the so-called exchange interaction [5]. The radical chain reaction requires a large amount of radicals in the flame plasma; it cannot be accommodated for living cells since radicals can burn the cell. Nevertheless, some bio-reactions like lipid peroxidation initiated by ROS proceed by radical chain mechanism [7]. Strong regulation of ROS concentration by enzymes (like superoxide dismutase, SOD) is crucially important for living cells. Cancer growth also can be dependent on a delicate balance in ROS concentrations [8]. It is important to stress that these balances are regulated by spin properties and depend on internal magnetic forces [2-8].
Two electrons on two πg MOs with antiparallel spins can produce also singlet excited states in O2 shown in Figure 1. Different distributions of two upper electrons on two highest occupied πg molecular orbitals provide (besides the ground triplet state ) two singlet excited states 1Δg and (Figure 1). The state 1Δg is doubly degenerate. It means there are two states with the same energy in Figure 1. This fact is very important for cancer PDT theory, but often ignored in practical applications.
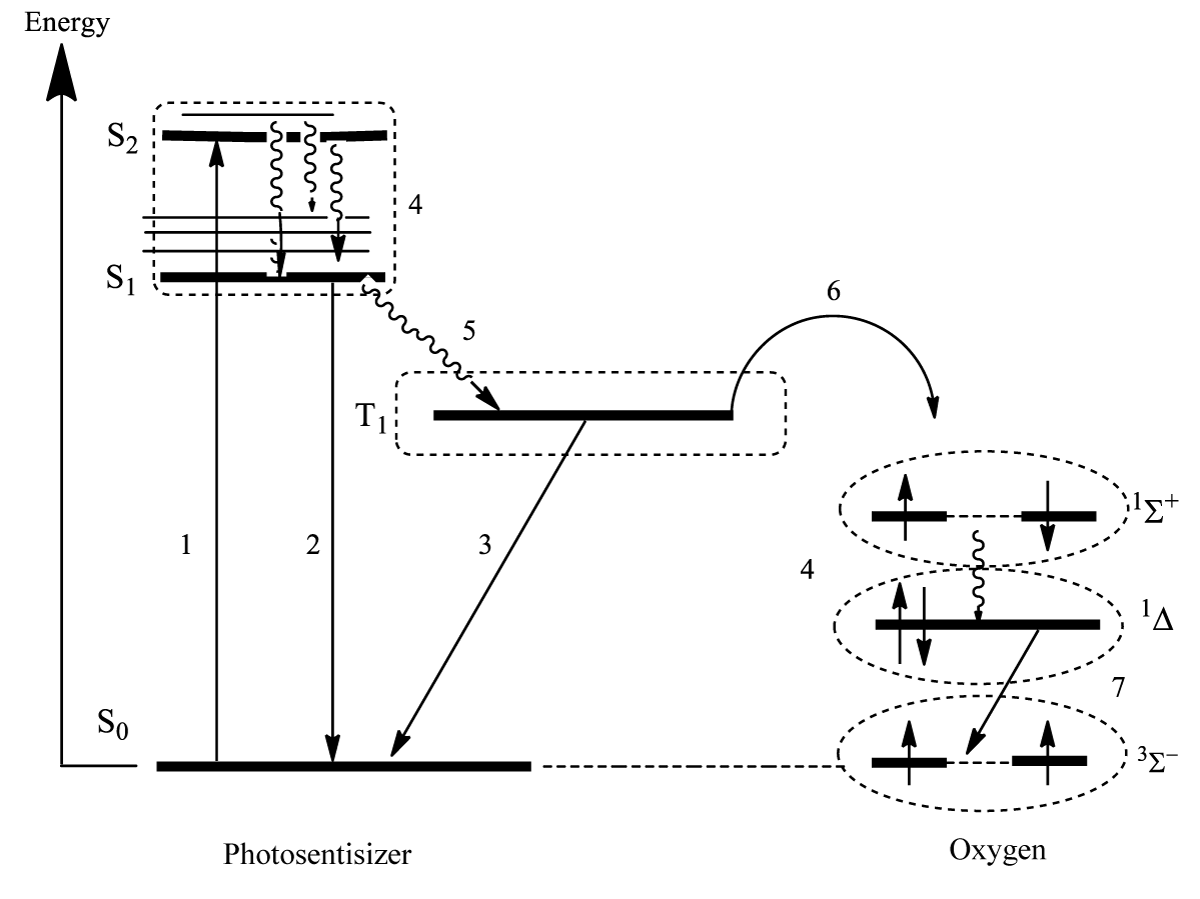
Cancerous tissue can be removed quite effectively by implementing a simple drug–the organic molecule of photosensitizer which needs to be elucidated by UV-visible light in the vicinity of the tissue [9]. This photodynamic therapy (PDT) utilizes the light source with an optical waveguide to produce photoexcitation of the drug. The excited photosensitizer being in contact with O2 molecules (always present in tissue) can transfer its electronic energy to O2 providing the singlet excited oxygen O2(1Δg), Figure 2. The second singlet excited state quickly relaxes transferring energy to the low-lying 1Δg state [9]. Internal relaxation between singlet states is spin-allowed.
Figure 2: PDT scheme. Light absorption (1), fluorescence (2), phosphorescence (3), internal conversion (4), intersystem crossing (5), energy transfer (6), oxygen emission at 1270 nm (7).
Electronic transition is strictly forbidden by spin and by orbital selection rules [2]. It is observed as a very weak near-infrared emission at 1270 nm with special sensitive photodetectors [9]. In a great difference from usual molecular spectra, this forbidden transition intensity is determined by weak magnetic interactions inside the O2 molecule [2] which are the focus of our review. The low rate of spontaneous oxygen emission 1Δg → determines the long lifetime (τΔ) of the singlet excited oxygen O2(1Δg) in the rarefied gas phase (about an hour). In dense gases (few seconds) and in solvents it is shortened; in water τΔ =3 μs [9]. This lifetime is enough to react with cancer tissue cells and kill them since the singlet excited oxygen has no spin restrictions for chemical reactions with singlet organic molecules. The cancer cells include highly unsaturated polymers and π-conjugated molecules, which are easy to be oxidized by O2(1Δg) species that possess an energy excess of 94 kJ/mole. The spin and energy are important factors of singlet oxygen reactivity with cancer tissues.
Light absorption by photosensitizer (PS) being in collision with an O2 molecule can lead to the charge-transfer (CT) excited state PS+ - O2- of the triplet spin nature (since O2 is triplet and PS is singlet in the ground states). This triplet radical pair (RP) 3(PS+• - O2-•) possesses an intrinsic magnetic force (IMF) which can induce the spin-flip in the superoxide ion-radical (O2-•), thereby providing the triplet-singlet (T-S) transition in this RP [6,7]. Thus the singlet RP has no spin restriction for chemical reactions with the tissue’s organic molecules. The singlet excited oxygen O2(1Δg) and superoxide ion-radical (O2-•) produced via the photosensitizer as a result of its electronic excitation are the main reactive oxygen species (ROS) which have no spin restrictions for organic oxidation in great contrast to the ground triplet state O2( ) oxygen which we breathe. The superoxide ion radical can be produced in dark biochemical reactions that accompany the electron transport chain (ETC) and other enzymatic redox processes [2,10]. Oxidative phosphorylation as the last step of cellular respiration, Eq.(1), provides the opportunity for superoxide leakage from the Krebs cycle. Thus, the random O2-• leakages always accompany normal metabolism. That is why the superoxide dismutase (SD) regulating superoxide concentration is the oldest enzyme on the Earth and occurred at the very beginning of aerobic evolution [1]. The lifetime of the ion-radical O2-• is comparable with τΔ in many biological liquids. These reactive oxygen species usually give rise to other ROS [1,7], like OH radicals (through the Fenton reaction [11]), OOH, or peroxynitrite [12]. All these processes depend on the spin and magnetic properties of the parent oxygen and its reduced derivatives which are highly reactive.
Singlet oxygen and some reactive oxygen species are often produced via the electronic excitation of photosensitizer dye (Figure 2). In the past two decades, tremendous efforts have been paid towards the design, synthesis, and development of new photosensitizer molecules for effective cancer treatment with PDT.
The PS typically is an organic molecule with the singlet ground state (S0); it possesses an even number of electrons paired with opposite spins as being located on the most energetically favorable molecular orbitals (MO). After absorbing light, one of the electrons is excited and transferred to a higher energy orbital with the spin orientation preserved (the first excited singlet state, S1) (Figure 2). PS in its singlet excited state is relatively unstable and can return to the ground state either by emission of photon (Fluorescence) or by release of the excitation energy in the form of chemical bond vibrations and heat dissipation (Internal Conversion, IC). Besides IC, the first excited singlet state can alternatively undergo intersystem crossing (ISC, electron spin reorientation) to the first excited triplet state T1. The driving force for the spin-flip during the ISC process is the internal magnetic interaction induced by spin-orbit coupling (SOC) in the PS electronic shell. It depends on the orbital reorganization during the S1 → T1 quantum transition [2]. SOC is higher when atomic orbital rotation (of the px-py type) accompanies the S1 → T1 transition. The T1 state lifetime is relatively long in the cell liquid (about a millisecond or longer) to enable either direct energy transfer (Type II) or electron transfer (Type I) reactions with oxygen resulting in ROS generation [13,14]. The quantum yield and relative contributions of these photochemical reactions depend on the PS structure, concentration of the PS, light intensity, and dose of molecular oxygen in the nearest micro-environment [14]. Most of the porphyrins generate mainly singlet O2(1Δg) oxygen through the Type II mechanism, while the palladium bacteriochlorophyll derivative is a pure generator of Type I superoxide ion products. Other bacteriochlorins generate both singlet oxygen and oxygen-centered radicals [13-17].
Optimization of the PS triplet T1 state is the main strategy currently employed in the photosensitizer design to increase their efficiency in ROS generation [13,15]. Introduction of heavy transition-metal ions, such as Pt(II), Ir(III), Zn(II), and Pd(II) into the porphyrin ring increases the ISC rate and enhances the yield of the long-lived triplet T1 state [13-16]. The metal ion choice can play a crucial role in the photochemical and redox properties of photosensitizers. It affects the spin density distribution in the triplet state of the PS molecule, which in turn influences its magnetic properties, ISC rate, and ability to perform an electron or energy transfer during ROS production according to Type I or Type II mechanisms. The oxidation potential of the metal ion is also an important factor determining the charge-transfer contribution to spin-orbit coupling (SOC) matrix element (ME) between S and T states [4,7]. The redox properties determine how easily the PS molecule can donate or accept electrons, which is important for the rate of energy transfer to oxygen molecules. Of course, SOC ME as an important magnetic parameter strongly depends on the atomic number (Z) of heavy metal ions (as Z4) [2]. However, the oxidation potential of the ion influences the atomic orbitals mixing and rotation through the CT contributions as was shown in the vase of τΔ calculation in singlet oxygen quenching by amines [18].
PDT mechanisms of tumor destruction
Various reactive oxygen species (ROS) being generated within the tumor tissue after its PS doping and irradiation are involved in the oxidation of numerous biological structures. The metastable O2(1Δg) molecules can be produced through PDT in such amounts which is sufficient to control various types of cancer cells without causing damage to healthy tissue. Some PS dyes are very effective in the high production of singlet O2(1Δg) oxygen through the Type II mechanism. Such natural dyes as Rose Bengal and various phthalocyanines served as useful PS drugs since the early days of PDT applications [17]. Illumination of PS induces the generation of the excited triplet T1 state (3PS*) which can transfer electrons, protons, or electronic energy, leading to the production of ROS. Numerous ROS cause apoptosis or necrosis of tumor cells by photochemical oxidation.
Modern PSs operate in both mechanistic Types I and II; they can lead to oxidative stress within irradiation time and determine the PDT anticancer activity, which consists of three main mechanisms: (1) effect on cancer cells, (2) effect on tumor blood vessels, (3) effect on the host immune system [15,17]. Compared to antibiotics, PDT does not develop bacterial resistance. Operating independently, these mechanisms have the potential to interact with one another; their contributions to the overall tumor response are currently under serious study [15,19,20]. The most significant features governing a given mechanism include the general parameters of the PS (its electronic structure, lipophilicity, size, and charge), its redox and photochemical properties, the tendency to aggregation, and the applied time intervals from the PS administration to irradiation (drug-to-light interval; DLI) [21,22]. A comprehensive combination of these factors is necessary to achieve optimal long-term tumor remission, particularly in the case of metastatic cancers. Different molecules have been approved for their clinical use as photosensitizers; they include the well-known porphyrin oligomer mixtures, such as Photofrin® or the Verteporfin® (Visudyne) and Temoporfin® (Foscan) porphyrins [20]. Hydrophilic compounds (e.g. padeliporfin, F2BOH, TPPS, AlPcS3) primarily bound to albumin and often exhibiting low stability, are commonly employed in vascular-targeted therapy (V-PDT, DLI ≤ 15 min). Conversely, hydrophobic compounds (e.g. Temoporfin, ZnPC) which are localized within the inner lipid core of lipoproteins, particularly low-density lipoproteins (LDL), are regarded to require a solubilizing carrier such as liposomes or polymeric micelles [19]. These compounds are typically employed in cellular-targeted photodynamic therapy (long-acting C-PDT, DLI ≥ 12 h) [20]. Some other PSs have entered different stages of clinical trials. Among the different families of metallophotosensitizers, Ru(II) complexes with azaheterocyclic ligands have become recently available [19]. Ruthenium(II) ion as a heavy element with a large Z4 value possesses a strong SOC-induced magnetic field being an efficient T1 state provider. Under illumination this PS produces various ROS being adsorbed near the intracellular membrane. Particular photosensitizers accumulate within specific organelles, while others may undergo redistribution over DLI time. Anyway, the primary intracellular targets for various PSs include the plasma membrane, endoplasmic reticulum, Golgi apparatus, lysosomes, and mitochondria. The singlet O2(1Δg) oxygen lifetime, its diffusion length and its DLI strongly depend on solvent microenvironment, pH, and organelle redox parameters. Spin-orbit coupling between 1Δg and states is forbidden in isolated oxygen molecules; thus, there is no magnetic force to induce transition in oxygen gas at near-zero pressure [2]. Internal magnetic interactions in the O2 molecule can be induced by charge-transfer admixtures to the wave functions of the 1Δg and states [18], thus generating the spin-flip and quenching the singlet excited O2(1Δg) oxygen. The SOC-induced mixing between and ... states is also very important for the O2(1Δg) state lifetime [2]. This SOC ME energy is relatively high (2.13 kJ/mole) and is dominative IMF for the whole ROS chemistry.
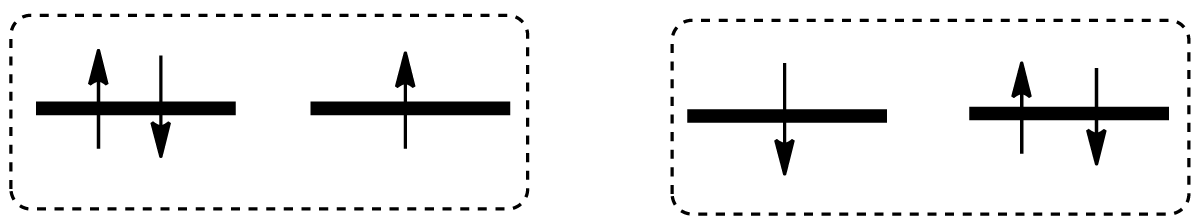
Superoxide ion-radical O2-• produced by Type I photo reactions from excited 3PS* possesses doubly degenerate ground states with two electronic configurations shown in Figure 3.
Figure 3: One electron is added to the ground state of oxygen providing two configurations of the O2-• ion. They differ by MS quantum number and by orbital angular momentum projections.
From the first glance both states in Figure 3 possess the same energy when we account for only Coulomb electric interaction. However, the account of intrinsic magnetic forces in the form of spin-orbit coupling provides a small energy difference (1.91 kJ/mole). This fine splitting was measured in the O2-• ionic beam by the electron time-of-flight spectroscopy [21]. Such a small energy is low compared to the dissociation of a chemical bond (300-500 kJ/mole) or even to the singlet O2 excitation (Figure 1). We must emphasize that the SOC energy can be used not as a brute chemical force, but as a “tiny key” whose gentle rotation “opens the door” to the ordinary organic chemistry of singlet states. Simply speaking, superoxide is produced from the excited 3PS* through the 3(CT) state of the triplet radical pair 3(PS+• - O2-•) [22,23]. Before dissociation this triplet RP has to undergo the T-S quantum transition to provide a fast chemical reaction allowed only in the singlet state. The intrinsic magnetic energy (1.91 kJ/mole) is high enough to induce a fast spin flip in the superoxide ion-radical O2-•, thereby providing the triplet-singlet (T-S) transition in this radical pair [2,7]. The strength of magnetic interaction 1.91 kJ/mole is an order of magnitude higher than typical SOC matrix elements for T-S transitions observed in organic dyes in the form of intense phosphorescence. For example, the efficient 3(nπ*)→S0 transition in formaldehyde is governed by SOC ME equal to 0.23 kJ/mole [2]. The square of the SOC ME value determines the rate of quantum T-S transition. Thus, we see that the T-S conversion rate constant in the superoxide radical pair is two orders of magnitude higher than the typical rate of the measured organic phosphorescence. These estimates convince us that the T→S conversion inside the radical pair (RP) must be fast enough to compete with the rate of RP decomposition into two radicals. Since the Type I mechanism of PDT with electron transfer to oxygen occurs with the participation of the photosensitizer adsorbed on the tumor, the transition to the singlet state covers the entire 3PS*-O2-cancer system. Now the tumor molecules in the singlet state are open to direct oxidation without spin inhibition. It is important to emphasize that the photosensitizer does not contribute to the SOC enhancement, it is restored and not consumed in such a PDT mechanism.
Possible pathways of PDT effect on cancer cells
The direct effect of ROS on cancer cells is to damage proteins, lipids, and DNA, resulting in an imbalance of homeostasis changes in cellular metabolism, and ion transportation [20]. The molecular response to oxidative stress consists of the activation of mitogen-activated protein kinase (MAPK) signal transduction pathways. They release several cytokines and mediators responsible for the processes of apoptosis, necrosis, and autophagy. These pathways are not mutually exclusive and can occur simultaneously in the same cell population. The interplay between apoptosis and necrosis after the PDT action is influenced by various accompanying factors. The most important parameters include the intracellular distribution of the PS, drug dose, light dose, DLI (influence the ISC and IC rate and total time of illumination), and the level of oxygen in the tissue. Moreover, there are some newly discovered types of regulated cell death important to PDT outcome, such as necroptosis [15], ferroptosis (cell death driven by iron-dependent lipid peroxidation) [19], pyroptosis (cell death triggered by inflammation) [21]. Activation of the programmed cell death can be induced either by the receptor or by mitochondrial pathways, where new superoxide ion-radicals can be additionally generated from enzymes of the Krebs cycle (besides the Type I mechanism of PDT). The death receptor pathway requires the presence of the so-called death receptors on the cell surface which, once combined with the proper ligand, initiate the caspase activation cascades leading to apoptosis [19]. The involvement of death receptor-dependent signaling is a consequence of cytokine expression by cells undergoing PDT-induced oxidative stress [17,24]. The mitochondrial apoptosis pathway is a very common mechanism in PDT since the primary target for many photosensitizers are these particular subcellular structures.
Technical comments. Two electronic configurations in Figure 3 differ by MS quantum number (in the left part MS =1/2, in the wright part MS =-1/2). Being in contact with the PS+• radical (with MS =1/2) the left state of the superoxide ion-radical produces the triplet state of the radical pair 3(PS+• - O2-•), while the Wright electronic configuration in Figure 3 produces the singlet state of the similar radical pair 1(PS+• - O2-•). The SOC ME between these T and S states includes the single-electron integral of the Lz operator between πgx-πgy orbitals, where Lz is an operator of the orbital angular momentum projection on molecular O-O axis. Such orbital integral provides the maximum possible value of the SOC matrix element for the oxygen atomic number Z=8. This SOC ME coincides with the observed SOC-induced splitting in the ground doublet state of the superoxide ion [21] according to theory [2]. A simple qualitative interpretation of these quantum-mechanical results looks like this. The T and S states of radical pair 3(PS+• - O2-•) and 1(PS+• - O2-•) differ by electron “jump” from πgx to πgy orbital with simultaneous spin-flip (Figure 3). Such electron “jump” corresponds to rotation of “electron cloud” around molecular axis; this rotation creates a magnetic field (magnetic torque) which induces the spin-flip [7,22].
In this mini-review, we have emphasized the role of spin-depending internal magnetic interactions in such reactive oxygen species as singlet O2(1Δg) oxygen and superoxide ion-radical O2-• generated in the course of photodynamic therapy. These two species, as many other ROS, are paramagnetic particles; the singlet state O2(1Δg) molecule – because of the orbital angular momentum, all others – because of non-paired spin. The necessity of ROS occurrences in aerobic life is determined by an evident paramagnetic nature of the O2( ) molecule. Oxygen from the air can easily oxidase paramagnetic metals but is unable to react with diamagnetic organic staff in the absence of radicals because of the spin prohibition. The oxygen molecule itself demonstrates outstanding magnetic property – the SOC-induced mixing between and states (Figure 1) which affects the entire photobiology of oxygen in the cell, including quenching of singlet O2(1Δg) and its phosphorescence.
Numerous dye photosensitizers upon illumination generate both types of ROS - O2(1Δg) and O2-• ion (as well as OH, OOH, NO, OONO-, H2O2). So far in PDT practice nobody paid attention to the outstanding magnetic property of the superoxide ion – the SOC-induced splitting of its ground doublet state. The observed energy of this splitting is relatively high and comparable with the SOC matrix element between the and states in the molecular ancestor O2; both magnetic parameters have the same origin - the symmetry properties of the πgx-πgy degenerate orbitals in the O2 system.
An important consequence of quantum perturbation theory, based on the above data, is that the energy of the SOC splitting in superoxide ion determines the rate of the triplet-singlet transition in the radical pair produced by an electron transferring from the triplet-excited photosensitizer to oxygen. This removes the spin prohibition for direct oxidation of membrane components or tumor organelles on which the 3PS* was adsorbed. This is possible since all active components, ROS, PS and tumor tissue arrange the common photo-reactive system.- Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11-19. Available from: https://doi.org/10.1016/j.tplants.2016.08.002
- Minaev BF. Electronic mechanisms of molecular oxygen activation. Russ Chem Rev. 2007;76(11):988-1010. Available from: https://www.russchemrev.org/RCR3720pdf
- Wigner E, Witmer EE. Zeits. F. Physik. 1928;51:859-864.
- Minaev BF, Minaeva VA. Spin-dependent binding of dioxygen to heme and charge transfer mechanism of spin-orbit coupling enhancement. Ukr Biol Acta. 2008;2(1):56-64. Available from: https://www.bioorganica.org.ua/UBAdenovo/pubs_6_2_08/Minaev_2008_2.pdf
- Zadeh-Haghighi H, Simon C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J R Soc Interface. 2022;19(193):20220325. Available from: https://doi.org/10.1098/rsif.2022.0325
- Prabhakar R, Siegbahn PE, Minaev BF. A theoretical study of the dioxygen activation by glucose oxidase and copper amine oxidase. Biochim Biophys Acta. 2003;1647(1-2):173-8. Available from: https://doi.org/10.1016/s1570-9639(03)00090-6
- Minaev BF. Magnetic torque in superoxide ion is the main driving force of dioxygen activation in aerobic life. Biomed J Sci Tech Res. 2021;38(4):121-131. Available from: http://dx.doi.org/10.26717/BJSTR.2021.38.006171
- Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol Lett. 2012 Dec;4(6):1151-1157. Available from: https://doi.org/10.3892/ol.2012.928
- Krasnovsky AJ, Jr. Primary mechanisms of photoactivation of molecular oxygen. History of development and the modern status of research. Biochemistry (Moscow). 2007;72:1065-1080. Available from: https://doi.org/10.1134/s0006297907100057
- Lane N. Oxygen: The Molecule that Made the World. Oxford: Oxford University Press; 2002. p. 365. Available from: https://books.google.co.in/books/about/Oxygen.html?id=LXPrLcFdIPwC&redir_esc=y
- Zakharov II, Kudjukov KYu, Bondar VV, Tyupalo NF, Minaev BF. DFT-based thermodynamics of Fenton reactions rejects the ‘pure’ aquacomplex models. Comput Theor Chem. 2011;964:94-99. Available from: https://doi.org/10.1016/j.comptc.2010.12.004
- Sukhina MC. Spin-orbit coupling induced intersystem crossing in peroxynitrite. XXVI Ukrainian Conference of Young Scientists; Cherkasy, 2024;158-161.
- Minaev BF. Photochemistry and spectroscopy of singlet oxygen in solvents. Recent advances which support the old theory. Chem Chem Technol. 2016;10(4):519-530. Available from: http://dx.doi.org/10.23939/chcht10.04si.519
- Foot C. Mechanisms of photosensitized oxidation: There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968;162:963-970. Available from: https://doi.org/10.1126/science.162.3857.963
- Riebe J, Bädorf B, Löffelsender S, et al. Molecular folding governs switchable singlet oxygen photoproduction in porphyrin-decorated bistable rotaxanes. Commun Chem. 2024;7:171. Available from: https://www.nature.com/articles/s42004-024-01247-7
- Loboda O, Tunell I, Minaev BF, Ågren H. Theoretical study of triplet state properties of free-base porphin. Chem Phys. 2005;312(1-3):299-309. Available from: http://dx.doi.org/10.1016/j.chemphys.2004.11.041
- Yashchuk LB. Possible electronic mechanisms of generation and quenching of luminescence of singlet oxygen in the course of photodynamic therapy: ab initio study. Biopolym Cell. 2006;22(3):231-235.
- Minaev BF. Spin-orbit coupling of charge-transfer states and the mechanism for quenching singlet oxygen by amines. Theor Exp Chem. 1984;20(2):199-201. Available from: https://link.springer.com/article/10.1007/BF00592809
- Pang Y, Li C, Deng H, Sun Y. Recent advances in luminescent metallacycles/metallacages for biomedical imaging and cancer therapy. Dalton Trans. 2022;51:16428-16438. Available from: https://doi.org/10.1039/D2DT02766F
- Dos Santos AF, de Almeida DRQ, Terra LF, Baptista MS, Labriola L. Photodynamic therapy in cancer treatment - an update review. J Cancer Metastasis Treat. 2019;5:25. Available from: https://doi.org/10.20517/2394-4722.2018.83
- Land JE, Raith W. Fine structure of O2− measured by electron time-of-flight spectroscopy. Phys Rev Lett. 1973;30:349-352. Available from: https://doi.org/10.1103/PhysRevLett.30.349.2
- Panchenko OO, Minaev BF. Enzymatic spin-catalysis in flavin-containing oxidases and magnetic orientation of birds. Cherkasy Univ Bull Biol Sci Ser. 2018;1:114-120. Available from: https://bio-ejournal.cdu.edu.ua/article/view/2804
- Minaev BF, Lunell S, Kobzev GI. Collision-induced intensity of the b1Σg+−a1Δg transition in molecular oxygen: Model calculations for the collision complex O2+H2. Int J Quantum Chem. 1994;50(4):279-292. Available from: https://doi.org/10.1002/qua.560500405
- Minaev BF. Quantum-chemical investigation of the mechanisms of the photosensitization, luminescence, and quenching of singlet 1Δg oxygen in solutions. J Appl Spectrosc. 1985;42(5):518-523. Available from: https://link.springer.com/article/10.1007/BF00661398