More Information
Submitted: July 02, 2024 | Approved: July 12, 2024 | Published: July 15, 2024
How to cite this article: Winn SP, Huang Y. Durable Response to Pembrolizumab and Lenvatinib in a Patient with Chemotherapy-refractory Cholangiocarcinoma. Arch Cancer Sci Ther. 2024; 8(1): 041-047. https://dx.doi.org/10.29328/journal.acst.1001043
DOI: 10.29328/journal.acst.1001043
Copyright License: © 2024 Winn SP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Distal cholangiocarcinoma; LEAP-005 trial; Pembrolizumab plus Lenvatinib
Durable Response to Pembrolizumab and Lenvatinib in a Patient with Chemotherapy-refractory Cholangiocarcinoma
Soe P Winn1* and Yiwu Huang2
1Department of Internal Medicine, Maimonides Medical Center, Brooklyn, NY, USA
2Department of Hematology-oncology, Maimonides Medical Center, Brooklyn, NY, USA
*Address for Correspondence: Dr. Soe P Winn, MD, Department of Internal Medicine, Maimonides Medical Center, Brooklyn, NY, USA, Email: [email protected]
Cholangiocarcinoma (CCA), a rare malignancy originating from bile duct epithelial cells, often presents a challenging prognosis due to its rarity, delayed diagnosis, and early recurrence post-curative-intent treatments. Additional complexities include difficulties in achieving R0 resection during surgical intervention and the lack of effective second-line treatments following the failure of first-line regimens, particularly in unresectable advanced cases.
In this case study, we demonstrate a durable response to a combination regimen of pembrolizumab and lenvatinib in a patient with distal CCA. Despite the regimen’s interim median Progression-Free Survival (PFS) of 6.1 months (95% CI, 2.1-6.4), our patient achieved a clinical and radiological PFS of approximately two years. The underlying mechanisms, potentially involving the upregulation of immune response pathways through undisclosed means or influenced by lenvatinib’s activation of T cells, might augment the sensitivity to PD-1 antibodies like pembrolizumab, contributing to the patient’s sustained response over two years.
This case also highlights the significance of the patient’s initial good health condition, multidisciplinary care, and the potential impact of molecular subtyping on treatment selection in a patient with distal CCA who underwent numerous diagnostic procedures, intricate surgical interventions, and subsequent treatment regimens over seven years. Additionally, we underscore significant landmark trials and emerging combination therapies, including chemotherapies, immunotherapy, and targeted treatments in this report.
Cholangiocarcinoma (CCA) is a rare type of cancer accounting for less than 1% of all cancer cases, primarily originating from the inner lining of the epithelial cells of the bile duct [1]. According to Patel, et al. analysis of SEER U.S cancer data statistics from 2001 to 2015 revealed an incidence rate of 1.26 cases of cholangiocarcinoma per 100,000 individuals annually. CCAs are now categorized into intrahepatic, and extrahepatic (perihilar and distal) CCAs based on their anatomic locations. According to recent data, intrahepatic cases are rising in incidence even though it has been argued that changes in ICD classification may have contributed to this trend with some extrahepatic CCAs potentially being misclassified as intrahepatic [2,3].
Recently, an association has been discovered between distal CCAs with certain risk factors that can induce chronic inflammation of the bile ducts. These factors include Primary Sclerosing Cholangitis (PSC), Inflammatory Bowel Disease (IBD), hepatolithiasis, choledochal cysts, or liver fluke infections, particularly in endemic Asian countries with Clonorchis sinensis and Opisthorchis viverrine are prevalent [4]. Furthermore, lifestyle factors and comorbidities such as diabetes, obesity, alcohol consumption, and smoking are known to elevate the risks of distal CCAs [2].
Despite its recognized risk factors, CCAs remain uncommon cancers, and, most of the cases present at locally advanced or late stages, often with unresectable or metastatic cancers. This is largely due to the vague nature of its early symptoms, such as right upper abdominal discomfort, malaise fatigue, etc. [5].
With the introduction of the 8th edition of the American Joint Committee on Cancer (AJCC) staging system, CCA is now classified into intrahepatic (iCCA) and extrahepatic (perihilar(pCCA), and distal(dCCA)) types, each with different TNM staging criteria based on their anatomical site of origin, characteristic, specific management approach, and prognosis [1,6]. Depth of tumor invasion has gained prognostic significance for survival with the new AJCC 8th edition classification. Additionally, the site of the tumor may influence survival outcomes, as dCCAs often present earlier clinical symptoms due to biliary obstruction [7,8].
Regrettably, early recurrence is frequently observed following pancreaticoduodenectomy for dCCA and the prognosis is still unfavorable despite recent advancements in management [7,9].
In this context, we aim to present a case of distal CCA in a patient who has achieved a long-term survival exceeding 7 years since the diagnosis of dCCA. We will also explore and discuss current recommendations for systemic therapy in metastatic CCA settings, along with the patient’s treatment regimens over the past 7 years.
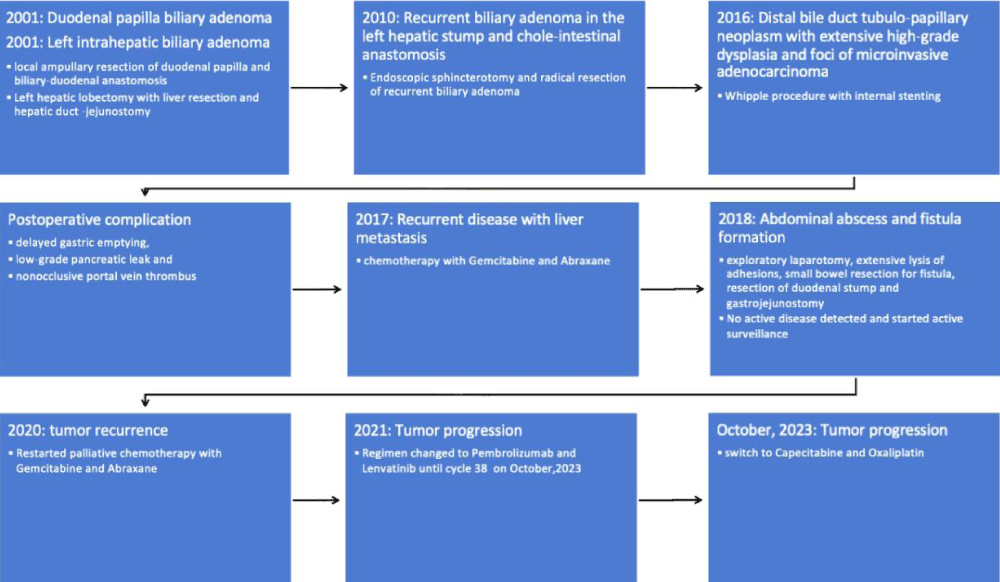
The patient is a 63-year-old female who was diagnosed with biliary adenoma in 2001 in China after she had a local ampullary resection of the duodenal papilla and reconstruction of the biliary/pancreatic duct with the duodenum. The patient was not obese and did not have diabetes. She is a non-smoker and non-alcoholic. There was no history of IBD, PSC, hepatolithiasis, or choledochal cysts, except for her residence in a liver fluke endemic area, in China. In the same year, 2001, she underwent left hepatic lobectomy with liver resection and hepatic duct-jejunostomy due to recurrent left intrahepatic papillary biliary adenoma.
In 2010, she underwent an endoscopic sphincterotomy and radical resection of the biliary adenoma in the left hepatic stump, followed by chole-intestinal anastomosis. Pathological examination revealed a papillary villiform adenoma accompanied by low and high-grade intraepithelial neoplasia in the left hepatic duct and extrahepatic bile duct, along with cholangiectasis, peri-cholangitis, and bile pigment stones. Subsequently, she immigrated to the US after the surgery and had been under surveillance.
On October 25, 2016, she underwent a Whipple procedure including pancreaticoduodenectomy and bilioenteric anastomosis with internal stenting due to a recurrent tumor within the distal bile duct in the pancreatic head. Pathology examination reported a 3.3 cm x 2 cm x 1.5 cm intraductal tubulo-papillary neoplasm with extensive high-grade dysplasia and foci of microinvasive adenocarcinoma. The surgical margins were negative for carcinoma and carcinoma invaded through the bile duct wall but didn’t show definitive invasion into the pancreatic parenchyma, perineural structures, or lympho-vascular system. None of the 27 nodes examined were positive for metastatic carcinoma. Pathological staging was pT2, pN0, stage IIA intrapancreatic distal common bile duct tubular-papillary neoplasm according to the current AJCC Cancer Staging Manual (8
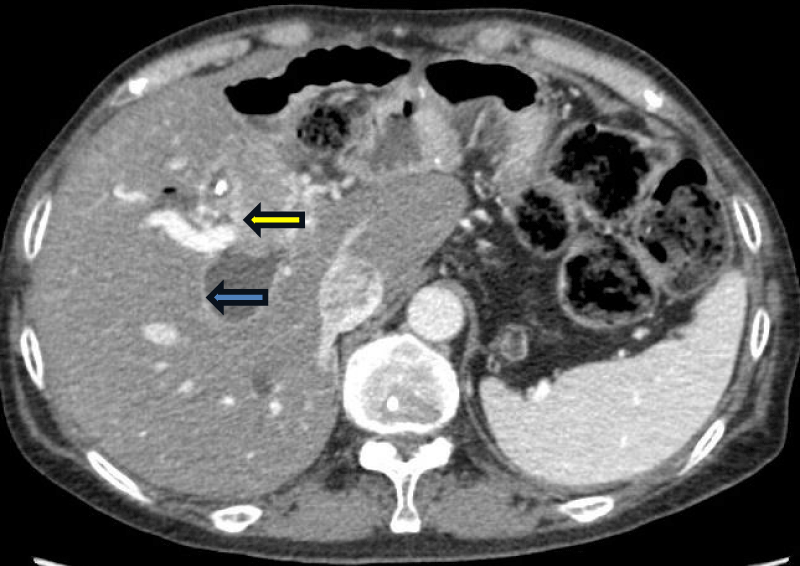
A follow-up CT scan on 6/9/17 revealed recurrent disease with liver metastasis (Figure 1). Chemotherapy with gemcitabine and Abraxane was initiated on 6/27/17 and continued for 12 cycles until 7/2018, at which point it was discontinued due to the development of an abdominal abscess and fistula formation. Subsequent treatment involved exploratory laparotomy, extensive lysis of adhesions, small bowel resection for fistula, resection of the duodenal stump, and gastrojejunostomy in 10/2018 after unsuccessful conservative management. No active disease was detected during surgery, prompting a recommendation for continued surveillance.
Figure 1: CT of the abdomen in transverse view on August 2021 showing a mass in the right intrahepatic bile duct (yellow arrow) associated with severe dilatation of the bile duct (blue arrow).
Next-Generation Sequencing (NGS) indicated no targetable gene mutation. A follow-up CT scan on 3/26/2020 revealed tumor recurrence, prompting the restart of palliative chemotherapy with gemcitabine and Abraxane from 6/2/2020 until 3/16/2021.
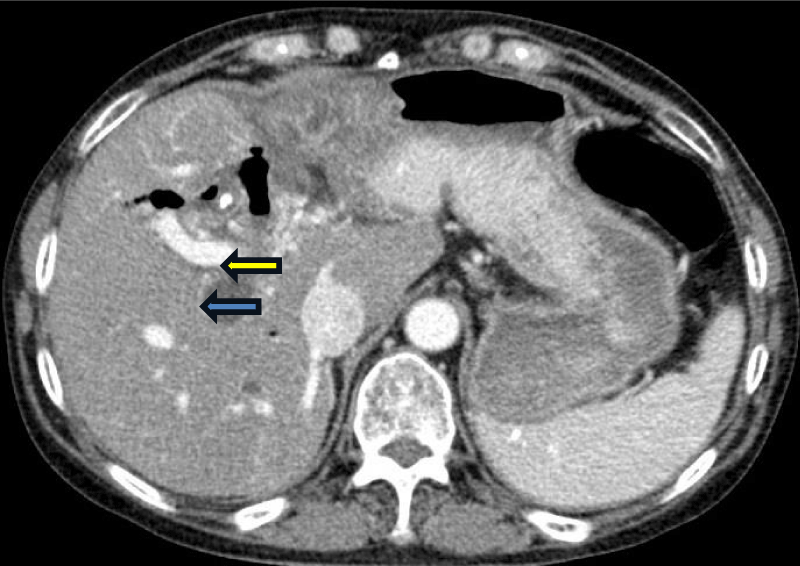
Subsequently, her chemotherapy regimen was switched to pembrolizumab and lenvatinib on 9/2/2021, due to disease progression noted in CT scans of the abdomen and pelvis, showing an increase in the size of a mass within the main right intrahepatic bile duct, associated with marked dilatation of the bile ducts (Figure 1). Initially, the patient tolerated the regimen well, and her tumor responded with a near-complete resolution of the mass and a marked decrease in dilatation of the right hepatic bile duct (Figure 2). However, due to toxicities including severe hypertension, QTc prolongation, severe fatigue, hypothyroidism, and diarrhea, lenvatinib required dose reduction and interruption, while pembrolizumab was continued every 3 weeks until October 31, 2023, totaling 38 cycles The patient was then hospitalized due to pancolitis and hepatitis, likely attributable to Pembrolizumab side effects. Her CT scan on October 10, 2023, revealed disease progression with the reappearance of a tumor mass near the right intrahepatic bile duct and biliary dilatation. Treatment was planned to switch to a capecitabine and oxaliplatin regimen, but the patient opted to continue treatment in China and subsequently lost follow-up. The timeline of events is summarized in Figure 3.
Figure 2: CT of the abdomen in transverse view on March 2023 showing near complete resolution of the tumor (yellow arrow) with marked improvement in dilatation of the right hepatic duct (blue arrow).
Figure 3: Timeline of events.
For the management of CCA, definitive surgery stands as the sole curative treatment. Prognostic factors like high TNM staging, histologic differentiation (poorly differentiated, mass forming, or periductal infiltrating types), vascular invasion, and perineural invasion all indicate early and high recurrence risks. According to DeOliveira et al., R0 resection margin and negative lymph node status are crucial for long-term survival, with 5-year survival rates marking 63, 30, and 27% for intrahepatic, perihilar, and distal tumors respectively [9]. However, due to the late presentation nature of the disease and a relapse rate exceeding 50% in previous curative-intent resected patients, adjuvant chemotherapies have been widely proposed [5,10,11].
Historically, the prognosis for unresectable advanced metastatic CCA has been dismal, with a median survival of approximately 3.9 months without any interventions [12]. Gemcitabine or 5-FU-based regimens were actively studied from early 2000 to 2010 with some studies showing improved outcomes [13-19]. The ABC-02 phase 3 trial comparing Gemcitabine & Cisplatin (GC) with gemcitabine alone showed improved median overall survival(OS): 11.7 vs. 8.1 months (Hazard ratio(HR) 0.64, 95% CI 0.52-0.80) [14]. Multicenter, open-label, randomized phase III trial comparing capecitabine plus oxaliplatin (CAPEOX or XELOX) with gemcitabine plus oxaliplatin (GEMOX) by Kim, et al. showed that 6-month Progression-Free Survival (PFS) was 46.7% for CAPEOX group vs. 44.5% for GEMOX group. Median OS and objective response between groups were not different. However, the efficacy of these regimens was limited with poor side-effect profiles, prompting the search for alternative treatment regimens in clinical trials such as gemcitabine plus nab-paclitaxel, or triple-drug regimens, GC plus nab-paclitaxel regimens(SWOG-1815 study), GC plus S-1(KHBO1401-MITSUBA), etc [20-22]. PRODIGE 38 AMEBICA phase 2 study comparing 5-FU, irinotecan, and oxaliplatin(mFOLFIRINOX) with the backbone GC regimen also failed to show benefit in terms of 6-month PFS (44.6% vs. 47.3%), median OS (11.7 vs. 13.8 months) with similar grade 3 or more adverse events (72.8% vs. 72%) [23].
In our patient’s case, she initially received the Gemcitabine plus Abraxane regimen from 6/27/17 to 3/16/2021. Even though there was an interruption of chemotherapy from July 2018 to June 2020 due to an abdominal abscess and fistula formation, our patient tolerated the chemotherapy well and no active cancer was detected during the surgery. This regimen is based on the phase 2 single-arm clinical trial on patients with advanced CCA. This study was based on the hypothesis that Paclitaxel’s inhibitory effect on cytidine deaminase enzyme which is one of the core enzymes for Gemcitabine’s metabolism thereby potentiating Gemcitabine delivery to the tumor cells [24]. PFS was 7.7 (95% CI, 5.4-13.1) months, and median OS was 12.4 (95% CI, 9.2-15.9) months with acceptable safety profiles which were comparable to the ABC-02 trial as described above [14,20].
Recently, immunotherapy with chemotherapy combination has emerged as the category 1 preferred regimen for advanced CCA, showing improved outcomes over traditional backbone regimens, GC regimen [1,25,26]. The first landmark novel trial is the TOPAZ-01 trial comparing GC plus durvalumab with GC plus placebo in a double-blind, placebo-controlled phase 3 trial involving 685 patients from 17 countries. Median OS was 12.8 (95% CI, 11.1-14.0) in the durvalumab group vs. 11.5 months (95% CI, 10.1-12.5) in the placebo group with HR 0.80, 95% CI 0.66-0.97. Median PFS was 7.2 months (95% CI, 6.7 to 7.4) in the durvalumab group vs. 5.7 months (95% CI, 5.6 to 6.7) in the placebo group [25]. Because of these significant improvements in terms of median OS and median PFS with similar grade 3 to 4 adverse events (75.7% vs. 77.8%) in both groups, the durvalumab with GC regimen as the 1st line treatment for the unresectable or metastatic advanced CCAs [1]. The second breakthrough trial is the KEYNOTE-966 trial comparing the GC plus pembrolizumab group with the GC plus placebo group in a randomized, double-blinded, placebo-controlled, phase 3 trial involving 1069 patients. The results were published in April 2023 that showed significant improvement in Median OS: 12.7 (95% CI, 11·5-13·6) in the pembrolizumab group vs. 10.9 months (95% CI, 9.9-11·6) in the placebo group (HR 0.83, 95% CI 0.72-0.95) [26]. By NCCN guidelines, an Immune checkpoint inhibitor (durvalumab or pembrolizumab) plus GC regimen is also recommended as the category 1 subsequent therapy for previously treated advanced CCAs patients who progress from non-immunotherapy-based systemic therapies.
In our patient’s case, she received pembrolizumab plus lenvatinib regimen after disease progression. That regimen was based on the ongoing phase II trial (LBA41; LEAP-005) for advanced CCA with disease progression after 1st line therapy. It is the combination of immunotherapy with a multi-targeted tyrosine kinase inhibitor (pembrolizumab+ lenvatinib). The interim result showed promising results, with an ORR of 9.7% (95% CI, 2.0-25.8%), and a median PFS of 6.1 months (95% CI, 2.1-6.4). Median OS was 8.6 months (95% CI, 5.6 to NR) [27]. The combination was first proposed based on preclinical studies demonstrating enhanced antitumor activities in mouse models through a combination of the anti-angiogenesis properties of Lenvatinib and the programmed cell death-1 blockage of Pembrolizumab. In their studies, Lenvatinib showed activation of interferon-gamma signaling pathway and immunomodulatory activities (decreasing tumor-associated macrophages and increasing CD-8+ T cells) which work synergistically with PD-1 antibody [28,29]. The FDA initially approved this combination for advanced endometrial carcinoma after promising results(longer PFS and OS) in phase 2 study-111-KEYNOTE-146 and phase 3 study-309-KEYNOTE-775 even in pMMR(mismatch repair: proficient) population [30,31]. The combination is also the first-line therapy for advanced renal cell carcinoma and is also currently investigated in other solid tumors such as EPOC1706, phase II trial for gastric and gastroesophageal junction adenocarcinoma and LEAP-005 study for advanced solid tumors (ovarian cancer, triple-negative breast cancer, gastric cancer, colorectal cancer, glioblastoma, biliary tract cancers, and pancreatic cancer) [32-34]. So, we have to follow up on the final result of the ongoing LBA41 LEAP-005 phase 2 trial.
For second-line treatment after the failure of first-line treatment of immunotherapy plus GC, no consensus or standardized treatment has been established and Active Symptom ControlASC) remains the standard of care due to discouraging results from the studies or minimal benefit over ASC [35]. The most notable phase 3 trial is the FOLFOX regimen (Folinic acid, Fluorouracil, and Oxaplatin) in the ABC-06 trial after 1st line treatment with GC. But, it only shows one month benefit over the active symptom control(ASC) arm; Median OS months of 6.2 months (95% CI 5.4-7.6) for the ASC+FOLFOX arm vs. 5.3 months (95% CI 4.1-5.8) for the ASC-alone arm [36]. There are 2 contradictory phase 2 trials, NIFTY and NALIRICC trials comparing liposomal irinotecan plus 5-FU and leucovorin (5-FU/LV) vs. 5-FU/LV in South Korea and Germany respectively. In the NIFTY trial, masked independent central review(MICR) showed the median PFS is 4.2 months (95% CI 2.8-5.3) in the irinotecan plus 5-FU/LV vs. 1.7 months (95% CI 1.4-2.6) in the 5-FU/LV group (HR: 0.61 (95% CI 0.44-0.86); p - value: 0.004) [37]. However, the NALIRICC trial conducted in Germany didn’t meet the primary end of PFS nor the secondary endpoint of OS or ORR. The median PFS is 2.64 months in the combined irinotecan plus 5-FU/LV group versus 2.3 months in the 5-FU/LV with HR 0.867, (95% CI 0.559-1.345) [38]. Thus, the incorporation of liposomal irinotecan to 5-FU/LV is not a plausible option as a second-line treatment regimen and that result also questions the PFS benefit seen in the NIFTY trial. Other trial results were seen in PRODIGE 38 AMEBICA phase 2 trial (Oxaliplatin, Irinotecan, and infusional Fluorouracil (mFOLFIRINOX) vs. Cisplatin and Gemcitabine (CISGEM/GC)), and REACHIN phase 2 trial (Regorafenib vs. placebo) are not encouraging as well [23,39].
Due to these unfavorable results with the second-line treatment and dismal prognosis, actionable targeted therapy for advanced CCAs that carry clinically relevant mutated gene targets is being actively researched. With the recent advances in comprehensive genetic profiling, studies have shown that more than 40% of advanced CCA patients harbor an actionable genetic mutation [40,41]. Therefore, molecular genetic testing for any actionable targets of genetic mutations (MSI-H/dMMR, TMB-H, NTRK gene fusion, BRAF V600E, Fibroblast Growth Factor Receptor (FGFR) fusion or rearrangement, HER2/neu overexpression and/or amplification, IDH1 mutation, RET gene fusion tests) are recommended for advanced CCA [1,41]. US FDA has approved Pembrolizumab or Dostarlimab-gxly for MSI-H/dMMR CCAs, Nivolumab plus Ipilimumab or Pembrolizumab for TMB-H CCAs, Larotrectinib and Entrectinib for the NTRK fusion-positive CCAs, Dabrafenib plus trametinib for BRAF V600E positive CCAs, Futibatinib or Pemigatinib for FGFR2 fusion or rearrangement CCAs, Ivosidenib for IDH1 positive CCAs, Trastuzumab plus Pertuzumab for the HER2/neu overexpressed/amplified CCAs and Selpercatinib and Pralsetinib for the RET fusion CCAs [1]. Although the FDA has approved several targeted therapies for these mutations, our patient lacked a targetable gene mutation in the NGS study, leading to a switch in treatment regimen with capecitabine and oxaliplatin as our next regimen.
Recently, a whole genome sequencing analysis of 500 CCA patients done by Jusakul, et al. showed a paradigm shift of whole CCA subtypes and classification at the molecular profiling level. They divided CCAs into 4 different clusters mainly by their genetic profile differences. Cluster 1 includes liver fluke infection, with hypermethylation of promoter CpG islands, ARID1A and BRCA1/2 mutations, and somatic promotor mutations. Cluster 2 includes mixed patients with liver fluke positive and negative infection with TP53 mutations and upregulated CTNNB1, WNT5B, and AKT1 gene expressions. Both cluster 1 and 2 has ERBB2 gene expression and mostly eCCAs. Both clusters 3 and 4 have liver fluke negative and iCCAs. Cluster 3 is upregulated with PD1, PDL2, and BTLA immune-related pathways. Cluster 4 has IDH1/2 and FGFR alterations and upregulations [42]. However, these findings need to be investigated further for their clinical implication and treatment decisions as some of these clusters will benefit from immunotherapy, IDH1, or FGFR-targeted therapies while others will not. Our patient likely belongs to the cluster 3 group characterized by upregulated immune-related pathways or may be influenced by lenvatinib’s activation of T cells, increasing sensitivity to PD-1 antibodies like pembrolizumab.
In summary, our patient has undergone treatment for advanced CCA since October 2016 and remains on active treatment as of the end of 2023. Throughout her cancer journey, she underwent multiple extensive surgeries, various chemotherapy regimens, and immunotherapy. As we discussed above, the efficacy of these treatments has shown varying degrees of benefit, with specific survival rates noted for each regimen. Despite this, her prolonged survival against CCA can likely be attributed to a combination of factors, including her initial good health status, the relatively non-aggressive nature of her tumor pathology, successful surgeries with clean margins (R0 resection), negative lymph node status, absence of vascular or perineural invasion, absence of distant metastasis and the continuous surveillance and active symptom control provided by our multidisciplinary team. Additionally, it is plausible that her tumor pathology, falling within cluster 3 may have contributed to increased sensitivity to immune checkpoint inhibitors, leading to prolonged survival against CCA. Further research and evidence may help to elucidate the underlying mechanisms driving her positive response to treatment.
Limitation
The patient’s pathology imaging results were unable to be obtained because the pathological slides were from China (out of state hospital in the U.S.) and there was no response from pathologists after multiple attempts to contact them.
Ethics statement
The authors declared they retain informed consent from the patient and approval from the head of the department to publish.
- Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127-140. Available from: https://doi.org/10.1016/j.annonc.2022.10.506
- Patel N, Benipal B. Incidence of Cholangiocarcinoma in the USA from 2001 to 2015: A US Cancer Statistics Analysis of 50 States. Cureus. 2019;11(1):e3962. Available from: https://doi.org/10.7759/cureus.3962
- Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848-854. Available from: https://pubmed.ncbi.nlm.nih.gov/22173164/
- Zheng S, Zhu Y, Zhao Z, Wu Z, Okanurak K, Lv Z. Liver fluke infection and cholangiocarcinoma: a review. Parasitol Res. 2017;116(1):11-19. Available from: https://pubmed.ncbi.nlm.nih.gov/27718017/
- de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341(18):1368-1378. Available from: https://pubmed.ncbi.nlm.nih.gov/10536130/
- Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13-21. Available from: https://pubmed.ncbi.nlm.nih.gov/22982100/
- Kim BH, Kim K, Chie EK, Kwon J, Jang JY, Kim SW, et al. Long-Term Outcome of Distal Cholangiocarcinoma after Pancreaticoduodenectomy Followed by Adjuvant Chemoradiotherapy: A 15-Year Experience in a Single Institution. Cancer Res Treat. 2017;49(2):473-483. Available from: https://pubmed.ncbi.nlm.nih.gov/27554480/
- Hong SM, Pawlik TM, Cho H, Aggarwal B, Goggins M, Hruban RH, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009;146(2):250-257. Available from: https://pubmed.ncbi.nlm.nih.gov/19628081/
- DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma. Ann Surg. 2007;245(5):755-762. Available from: https://journals.lww.com/annalsofsurgery/abstract/2007/05000/cholangiocarcinoma__thirty_one_year_experience.13.aspx
- Komaya K, Ebata T, Shirai K, Ohira S, Morofuji N, Akutagawa A, et al. Nagoya Surgical Oncology Group. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 2017;104(4):426-433. Available from: https://pubmed.ncbi.nlm.nih.gov/28138968/
- Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97(1):56-64. Available from: https://pubmed.ncbi.nlm.nih.gov/19937985/
- Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver. 2009;3(4):298-305. Available from: https://pubmed.ncbi.nlm.nih.gov/20431764/
- Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469-474. Available from: https://pubmed.ncbi.nlm.nih.gov/20628385/
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. Available from: https://pubmed.ncbi.nlm.nih.gov/20375404/
- Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15(3):478-483. Available from: https://pubmed.ncbi.nlm.nih.gov/14998852/
- Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, et al. EORTC Gastro Intestinal Tract Cancer Group. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41(3):398-403. Available from: https://pubmed.ncbi.nlm.nih.gov/15691639/
- Halim A, Ebrahim MA, Saleh Y. A phase II study of outpatient biweekly gemcitabine-oxaliplatin in advanced biliary tract carcinomas. Jpn J Clin Oncol. 2011;41(2):217-224. Available from: https://pubmed.ncbi.nlm.nih.gov/21062755/
- Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95(7):848-52. Available from: https://pubmed.ncbi.nlm.nih.gov/16969352/
- Jang JS, Lim HY, Hwang IG, Song HS, Yoo N, Yoon S, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol. 2010;65(4):641-7. Available from: https://pubmed.ncbi.nlm.nih.gov/19652971/
- Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, et al. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4(12):1707-1712. Available from: https://pubmed.ncbi.nlm.nih.gov/30178032/
- Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5(6):824-830. Available from: https://pubmed.ncbi.nlm.nih.gov/30998813/
- Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, et al. Kansai Hepatobiliary Oncology Group (KHBO). Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30(1):102-110. Available from: https://pubmed.ncbi.nlm.nih.gov/35900311/
- Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, et al. PRODIGE 38 AMEBICA Investigators/Collaborators. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J Clin Oncol. 2022;40(3):262-271. Available from: https://pubmed.ncbi.nlm.nih.gov/34662180/
- Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012 ;2(3):260-269. Available from: https://pubmed.ncbi.nlm.nih.gov/22585996/
- Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. N Engl J Med Evidence. 2022;1(8). Available from: https://evidence.nejm.org/doi/10.1056/EVIDoa2200015
- Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853-1865. Available from: https://pubmed.ncbi.nlm.nih.gov/37075781/
- Villanueva L, Lwin Z, Chung HC, Gomez-Roca C, Longo F, Yanez E, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J Clin Oncol. 2021 ;39(3_suppl):321-321. Available from: https://ascopubs.org/doi/10.1200/JCO.2021.39.3_suppl.321
- Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019 Feb;14(2). Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0212513
- Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993-4002. Available from: https://pubmed.ncbi.nlm.nih.gov/30447042/
- Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020 Sep 10;38(26):2981-2992. Available from: https://pubmed.ncbi.nlm.nih.gov/32167863/
- Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Study 309–KEYNOTE-775 Investigators. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022;386(5):437-448. Available from: https://pubmed.ncbi.nlm.nih.gov/35045221/
- Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289-1300. Available from: https://pubmed.ncbi.nlm.nih.gov/33616314/
- Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057-1065. Available from: https://pubmed.ncbi.nlm.nih.gov/32589866/
- Lwin Z, Gomez-Roca C, Saada-Bouzid E, Yanez E, Longo Muñoz F, Im SA, et al. LBA41 LEAP-005: Phase II study of lenvatinib (len) plus pembrolizumab (pembro) in patients (pts) with previously treated advanced solid tumours. Ann Oncol. 2020;31(Suppl 4). Available from: https://www.annalsofoncology.org/article/S0923-7534(20)42353-1/fulltext.
- Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328-2338. Available from: https://pubmed.ncbi.nlm.nih.gov/24769639/
- Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690-701. Available from: https://pubmed.ncbi.nlm.nih.gov/33798493/
- Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22(11):1560-1572. Available from: https://pubmed.ncbi.nlm.nih.gov/34656226/
- Vogel A, Wenzel P, Folprecht G, Schütt P, Wege H, Kretzschmar A, et al. 53MO Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabine-based therapies (NALIRICC – AIO-HEP-0116). Ann Oncol. 2022;33(Suppl). Available from: https://www.annalsofoncology.org/article/S0923-7534(22)01932-9/fulltext
- Demols A, Borbath I, Van den Eynde M, Houbiers G, Peeters M, Marechal R, et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann Oncol. 2020;31(9):1169-1177. Available from: https://pubmed.ncbi.nlm.nih.gov/32464280/
- Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res. 2018 Sep 1;24(17):4154-4161. Available from: https://pubmed.ncbi.nlm.nih.gov/29848569/
- Silverman IM, Murugesan K, Lihou CF, Féliz L, Frampton GM, Newton RC, et al. Comprehensive genomic profiling in FIGHT-202 reveals the landscape of actionable alterations in advanced cholangiocarcinoma. J Clin Oncol. 2019 ;37(15_suppl):4080-4080. Available from: https://ascopubs.org/doi/10.1200/JCO.2019.37.15_suppl.4080
- Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116-1135. Available from: https://pubmed.ncbi.nlm.nih.gov/28667006/