More Information
Submitted: August 27, 2022 | Approved: September 19, 2022 | Published: September 20, 2022
How to cite this article: Rodríguez MC, Báez GB, Luna YH, Fernández DRH, Palomo AG, et al. The combination of very-small size proteoliposomes and alum is a safe adjuvant alternative for inducing anti-EGF antibodies: a preclinical study. Arch Cancer Sci Ther. 2022; 6: 018-030.
DOI: 10.29328/journal.acst.1001029
Copyright License: © 2022 Rodríguez MC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: VSSP; Alum; Montanide; hrEGF-p64; Cancer vaccine; PAbs response; Toxicity; Neutralizing potential
Abbreviations: CY: Cyclophosphamide; DCs: Dendritic Cells; EGFR: Epidermal Growth Factor Receptor; ERK1/2, Extracellular Signal-Regulated Kinase 1/2; FITC: Fluorescein Isothiocyanate; HER: Epidermal Growth Factor Receptor; hrEGF: Human recombinant Epidermal Growth Factor; IFNγ: Interferon-Gamma; IL-4: Interleukin 4; MAbs: Monoclonal Antibodies; MAPK: Mitogen-Activated Protein Kinase; MDSC: Myeloid-Derived Suppressor Cells; NH4SCN: Ammonium Thiocyanate; NSCLC: Non-Small Cell Lung Cancer; PAbs: Polyclonal Antibodies; Th: T-helper; TKIs: Tyrosine Kinase Inhibitor; VSSP: Very Small Size Proteoliposomes
The combination of very-small size proteoliposomes and alum is a safe adjuvant alternative for inducing anti-EGF antibodies: a preclinical study
Mabel Cruz Rodríguez1, Gretchen Bergado Báez2, Yerandy Hechevarría Luna3, Diana Rosa Hernández Fernández2, Addys González Palomo2, Narjara González Suárez4, Carlos Yordan González Castillo2, María del Carmen Luzardo Lorenzo3, Lisset Chao García2 and Belinda Sánchez Ramírez2*
1Department of Experimental Preclinical Research in Thoracic Tumors, Bellvitge Biomedical Research Institute (IDIBELL), Barcelona, Spain
2Immunology and Immunotherapy Direction, Center of Molecular Immunology, Havana, Cuba
3Proteins Study Center, Faculty of Biology, University of Havana, Havana, Cuba
4Molecular Oncology Laboratory, Department of Chemistry, University of Quebec in Montreal, QC, Canada
5EPOVAC Direction, Center of Molecular Immunology, Havana, Cuba
*Address for Correspondence: Belinda Sánchez Ramírez, Immunology and Immunotherapy Direction, Center of Molecular Immunology, Havana, Cuba, Email: [email protected]
Immunization with human recombinant EGF chemically bound to the P64k protein of Neisseria meningitides (hrEGF-P64k) and adjuvanted in Montanide ISA 51 VG (Montanide) is an efficient strategy to induce polyclonal antibodies (PAbs) response targeting this self -antigen in cancer patients, which is the basis of the CIMAvax-EGF vaccine. The neutralizing potential of EGF-specific induced PAbs supports promising clinical data obtained to date with this vaccine. Herein, we evaluated a combination of very small-size proteoliposomes (VSSP) and aluminum hydroxide (Alum) as a novel adjuvant to induce specific PAbs with neutralizing and anti-proliferative properties on tumor cells, considering EGF as a model antigen. Toxicity at the injection site was not detected for the vaccine formulation containing VSSP/Alum, and it was immunogenic in BALB/c mice, as evidenced by the induction of high titers of EGF-specific polyclonal antibodies (PAbs). While schedule optimization increased the magnitude of the PAbs response induced by VSSP/Alum, induced PAbs’s avidity and intrinsic neutralizing potential were comparable to the humoral response induced by Montanide. Also, VSSP addition switched IgG subclasses distribution into a Th1-like pattern, as obtained with Montanide and desirable for a cancer vaccine. Finally, equivalent PAbs titers were induced by the vaccine formulations adjuvanted in VSSP/Alum or Montanide in tumor-bearing-mice, and immunosuppressed mice, suggesting the feasibility of the VSSP/Alum combined adjuvant for inducing anti-EGF antibodies in cancer patients at advanced stages of the disease.
The Epidermal Growth Factor Receptor (EGFR) is a widely validated Tumor-Associated Antigen (TAA), whose overexpression correlates with bad prognosis and resistance to conventional antitumor therapies [1]. The interaction with specific ligands promotes receptor activation mediated by dimerization with related HER receptors, and further activation of a complex signaling network, which stimulates tumor cell proliferation and survival [2]. Among multiple therapeutic approaches developed to block ligand-induced EGFR signaling, some specific tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (MAbs) have reached approval for the treatment of several malignancies, including non-small cell lung cancer (NSCLC), head and neck or colorectal carcinoma, with encouraging results [3]. EGF is a prominent ligand for this receptor, tightly related to its oncogenic potential [4], where high levels of circulating EGF correlate with poor prognosis in NSCLC, gastric carcinoma, melanoma, and multiform glioblastoma [5].
Active immunotherapies targeting both EGFR and/or their ligands have been addressed and evaluated in preclinical and clinical settings [6]. In this regard, CIMAvax-EGF is known as the first registered therapeutic vaccine in Cuba, as well as the first registration of a lung cancer vaccine in the world, approved as a switch maintenance therapy in several countries [7]. The phase III trials reported a clinical benefit for patients with high EGF serum concentration and treated with the vaccine, being the castration of circulating EGF by immunization-induced PAbs the main action mechanisms described for CIMAvax-EGF [8].
Therapeutic cancer vaccines pursue the challenge of stimulating an exhausted immune system (due to treatment toxicity or aging of the patients), and - in the case of vaccines based on EGFR and its ligands - overcoming tolerance to self-antigens. Accordingly, the selection of a strongly immunogenic adjuvant gains relevance in the design of cancer vaccines [9]. Montanide ISA 51 VG is the current adjuvant of CIMAvax-EGF. This stable water-in-oil emulsion generates a deposit at the injection site, eliciting high and long-lasting specific antibody titers with neutralizing capacity [10]. Despite demonstrated safety of the vaccine, pain at the site of injection due to Montanide accumulation upon chronic use is reported among the most frequent adverse events [8]. This background propels the search for innovative and safe adjuvants for this one - and extended to other cancer vaccines- without compromising the magnitude and quality of the induced PAbs response.
It has been recently proposed that the use of combined adjuvants may result in a synergistic increase in the immune response [9]. Different adjuvants induce multiple and complementary mechanisms that include maturation of DCs (dendritic cells) with the consequent expansion of CTLs and/or antibody-secreting B cells, as well as disruption of tumor immunosuppression mechanisms [9]. Accordingly, we proposed a combination of aluminum salts (Alum) with VSSP derived from the outer membrane of Neisseria meningitides, with attractive properties as an adjuvant for cancer vaccines [11] and immunomodulator [12], as an alternative to Montanide to induce anti-EGF antibodies. Since systemic toxicity of the current vaccine formulation has been characterized in preclinical models, where evidence of damage was related to local inflammation at the injection site [13], while VSSP-adjuvanted vaccines do not show signs of local or systemic damage [7], local toxicity was characterized in immunized mice. Our results suggest that the combination of both adjuvants elicits high titers neutralizing PAbs, with a similar th-associated IgG subclasses pattern to the current formulation, and without evidences of toxicity at the injection site.
Antibodies and reagents
For Western Blot experiments, antibodies specific to phosphorylated EGFR (Tyr1068) and β-actin, as well as anti-rabbit IgG-HRP conjugate were purchased from Cell Signaling. Human recombinant EGF was obtained from the Genetic Engineering and Biotechnology Institute. The TKI AG1478 used as control, as well as the ammonium thiocyanate used in avidity assays, were acquired from Sigma. Human EGF conjugated to fluorescein isothiocyanate (FITC) used for flow cytometry assays (FACS) was acquired from Dako.
Cell lines
Human epidermoid carcinoma A431 (ATCC CRL1555), human lung adenocarcinomas H125 (CRL-5801) and F3II mammary carcinoma were obtained from the American Type Tissue Culture Collection (ATCC). Cells were maintained in Dubbelco´s minimum essential medium DMEM-F12 (Gibco) supplemented with 10% fetal calf serum (FCS) (Gibco).
Antigen, adjuvants and immunogen preparation
The antigen hrEGF-P64k conjugate was produced at the Center of Molecular Immunology and administered at a dose of 63 µg, which is equivalent to 10 µg of EGF. Montanide ISA 51 VG was obtained from SEPPIC (France). VSSP was produced at the Finlay Vaccine Institute and the Center of Molecular Immunology. Alum was purchased from Sigma.
When Montanide ISA 51 was used as adjuvant, hrEGF-P64k conjugate was mixed with an equal volume of the adjuvant until emulsification. In the cases where Alum was used as an adjuvant, the conjugated was incubated with 1 mg of Alum under gentle stirring at room temperature, for 30 min. For the evaluation of the combined adjuvant VSSP/Alum, the antigen was incubated with 200 µg of VSSP and 1 mg of Alum under stirring at room temperature, for 30 min.
Immunization protocols
Female BALB/c and C57/BL6 mice, aged 8 - 12 weeks old, were obtained from the National Center for Laboratory Animals Production (CENPALAB, Havana, Cuba). Mice were kept under pathogen-free conditions. Animal experiments were approved by the Center of Molecular Immunology’s Institutional Animal Care and Use Committee.
Evaluation of tissue damage at the inoculation site
BALB/c mice (n = 5 for each group) were immunized once with 63 μg of hrEGF-P64k in different adjuvants: VSSP/Alum (200 μg/1 mg, sc.); VSSP ((200 μg, sc.); Alum (1 mg, sc) or Montanide ISA 51 VG (v: v, im.). The mice were sacrificed and muscles tissues samples from the inoculation and adjacent sites were removed. The tissue fragments were fixed in neutral buffered formalin (10%) and embedded in paraffin following the standard histological procedures. Hematoxylin-eosin staining was done. Histological evaluation was performed using digital imaging with optical microscopy (Olympus BX41). Here, infiltration of lymphocytes, inflammatory infiltration, and invasion of tissue parameters was analyzed.
Evaluation of hrEGF-P64k immunogenicity in alterna-tive adjuvants in healthy mice
BALB/c mice (n = 5 for each group) were immunized with 63 μg of hrEGF-P64k in different adjuvants: VSSP/Alum (200 μg/1 mg, sc); Alum (1 mg, sc) or Montanide ISA 51 VG (v: v, im).
Each administration of the vaccine formulations was distributed at two inoculation sites. A first scheme defined as “induction-phase” was integrated by four biweekly spaced immunizations and a re-stimulation dose after three-month. On days -2, 35, 56, 72, 96, 102, 124, 146, and 162, blood was taken to obtain serum. A second scheme defined as a “dose-maintenance schedule” was composed of four biweekly spaced immunizations and three monthly-spaced immunizations. On days -2, 35, 56, 84, 112 and 144 blood samples were extracted to obtain serum.
Sera obtention from blood
Blood samples were incubated at 37 °C for 30min and then at 4 °C for one hour. Finally, samples were centrifuged at 3000 g for 10 min and sera were collected and preserved at -20 °C.
PAbs recovery from immune sera
Pooled immune sera from each immunization group were diluted 1/5 in PBS and IgG Isotype PAbs were isolated by Protein a affinity chromatography. After elution, PAbs were dialyzed and stored in PBS at -20 °C.
Evaluation of hrEGF-P64k immunogenicity in alterna-tive adjuvants in immunocompromised mice
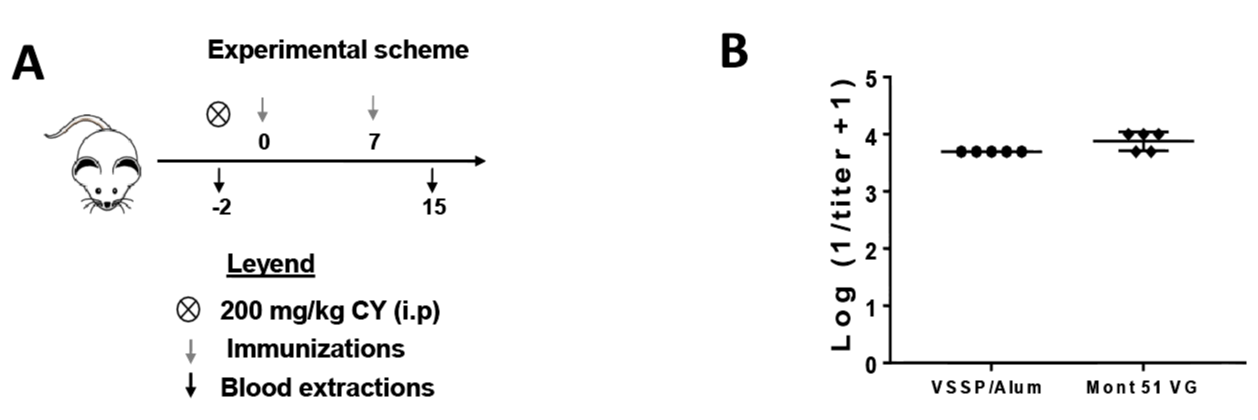
Immunogenicity of alternative vaccine formulations after administration of cyclophosphamide: BALB/c mice (n = 5 for each group), on day -2 were inoculated with an intraperitoneal (i.p) dose of cyclophosphamide (CY) (200 mg/kg of weight, in PBS). On days 0 and 7, mice were immunized with 63 μg of hr EGF-rP64k adjuvanted in VSSP/Alum (200 μg/1 mg, sc) or Montanide ISA 51 VG (v: v, im). On days 0 and 15 blood samples were taken from immunized animals to obtain serum.
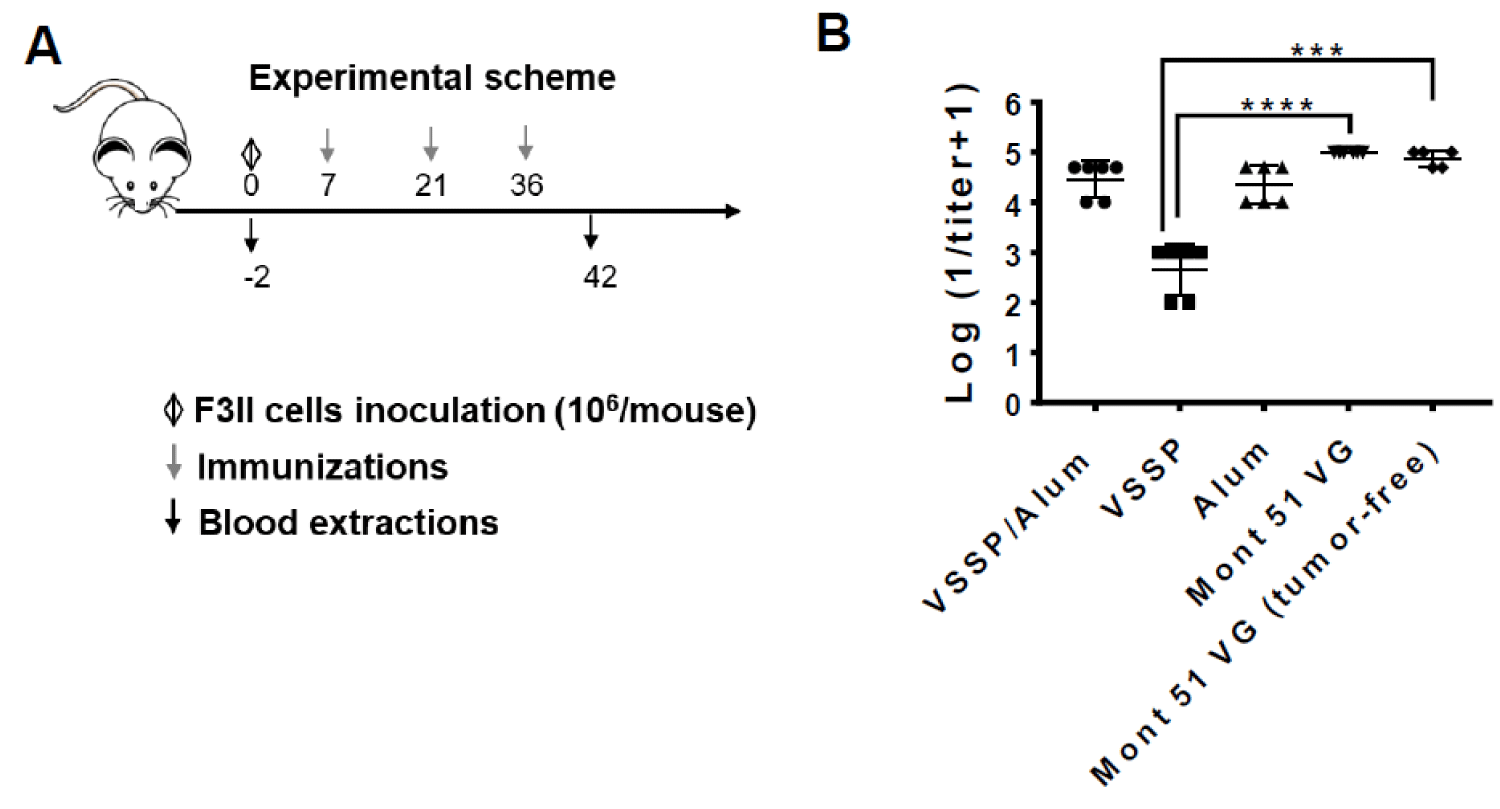
Immunogenicity of alternative vaccine formulations in F3II tumor-bearing mice: Alternatively, a total of 20 BALB/c mice, were inoculated with 106 F3II cells in the right flank, subcutaneously. After 10 days, tumor burden was confirmed and mice were randomized into four groups (n = 5 for each group). Next, three biweekly spaced immunizations with hrEGF-P64k (63 μg) adjuvanted in VSSP/Alum (200 μg/1 mg, sc), VSSP (200 μg, sc), Alum (1 mg, sc) or Montanide ISA 51 VG (v: v; im) were performed. A control group of mice were not challenged with tumor cells but received hr-EGF-p64 adjuvanted in Montanide, included as a control. A week after the last immunization mice were sacrificed and immune serum was isolated from the blood. Pre-immune serum was taken on day -2 of the scheme.
ELISA
Titration of EGF-specific IgG-isotype PAbs: Microtiter plates (High binding, Costar, USA) were coated with 10 μg/mL of hrEGF in carbonate buffer, 0.1 M, pH 9.6, and incubated overnight at 4 °C. Plates were blocked with FCS 2% in PBS/Tween 20 0.05%. Serial dilutions of the immune sera (ranging from 1:102-1:106) were incubated for 1 h at 37 °C for PAbs titration. Pre-immune sera diluted 1:102 was included for each group. Alkaline phosphatase-conjugated anti-mouse-IgG obtained in goat (1:1000) was added and incubated for 1 h at 37 °C. After the addition of p-nitrophenyl phosphate (1 mg/mL) (Sigma) in diethanolamine buffer pH 9.8, the absorbance at 405 nm was measured using a microwell system reader (Organon Teknica, Salzburg, Austria).
Titration of subclasses of EGF-specific IgG-isotype PAbs
For IgG subclasses titration, after incubation with dilutions of the immune sera, as previously described, mouse-specific IgG1, IgG2a, or IgG2b biotinylated antibodies were added (1:4000), followed by alkaline phosphatase-conjugated anti-rabbit IgG (1/4000). Pooled sera from each group corresponding to day 35 of the induction scheme were used.
Avidity determination
For avidity determination, purified PAbs (100 μg/mL) was added to EGF-coated plates and incubated for 1 hour at 37 °C.
Subsequently, indicated concentrations of the chaotropic agent ammonium thiocyanate (NH4SCN) in the range of 0 M to 4 M were applied. Detection of IgG was performed as described above. Sera from each group corresponding to day 140 of the induction scheme were used.
Flow cytometry
Pooled sera from each group corresponding to day 124 of the induction doses scheme, were diluted at 1:100 and incubated with EGF-FITC (0.2 μg/mL) for 30 min at 37 °C. Subsequently, A431 cells were incubated with these preparations for 20 min at 4 °C. A431 cells incubated with pre-immune sera (1:100) and EGF-FITC were included as a specificity control. Unlabeled cells and cells incubated only with EGF-FITC were considered as negative and positive controls for the staining, respectively. All the samples were characterized attending to mean fluorescence intensity (MFI) using a Gallios flow cytometer (Beckton Dickinson, San Jose, CA, USA), and FlowJo 7.6 software was used for analysis.
Western blot
H125 or A431 cells were grown to 70% of confluence in 12-well plates (Greiner) and starved in serum-free DMEM for 12 h.
Pools of immune sera from each group (1/100) at indicated extraction days, or pre-immune serum included as a negative control, were mixed with EGF (100 ng/mL) and pre-incubated for 30 min at 37 °C. Tyrosine kinase inhibitor AG1478 (10 μM) was added to the cells 15min prior to stimulation with EGF, as a control for EGFR inhibition. Cells were pulsed with the mixtures or controls for 10 min, then lysed, and phosphorylated EGFR was detected by immunoblotting with specific antibodies. Unstimulated cells were included as a negative control. Structural protein (beta-actin) levels were also evaluated as a loading control.
Viability assays
A431 (1.5 × 103) cells were seeded in flat-bottomed 96-well plates in DMEM-F12 supplemented with 10% FCS. Twenty-four hours later culture medium was removed and cells were starved in DMEM without FCS for 16 hours. To evaluate the impact of PAbs induced by vaccination on cell viability, cells were next maintained in DMEM supplemented with 0.001 nm of EGF in presence of purified PAbs obtained from immune sera of each group (0.25 μg/mL) at day 140 of the maintenance scheme. After 72 h of incubation, cell viability was determined by the modified colorimetric MTT assay. Formazan crystals were dissolved in DMSO, absorbance was measured at 540 nm, and the reference wavelength (was 620 nm). The absorbance of the culture medium was considered as background control. Cells incubated with PAbs purified from pre-immune sera, in presence of stimulating concentration of EGF (0.001 nm) were included as specificity, while cells incubated only with EGF (0.001 nm) were considered as maximum viability control. In these experiments, TKI AG1478 was used as cytotoxicity control. Cell viability was determined according to the next formula:
Cell viability (%) = [∆(A540nm-A630nm) treated cells/∆(A540nm-A630nm) maximum viability control] x 100
Statistical and graphical analysis
GraphPad Prism program (version 7.0) was used for the graphical analysis of the results. Statistical analysis was performed using the IBM SPSS Statistic 21 program. GraphPad Prism software. Densitometric analysis of the blots was performed with the ImageJ software. Normality and variance homogeneity of the data were analyzed by Shapiro-Wilk’s and Levene’s tests, respectively. Kruskal-Wallis test and Dun post-test were used to determine statistical differences when we didn’t obtain samples normality or/and variance homogeneity. Alternatively, one-way ANOVA and multiple comparison post-tests were applied. In the analysis of the kinetics of EGF-specific PAbs titers, to evaluate the differences among groups or extraction days, a linear generalized model was applied, using “time” and “group” as factors. Significant differences were emphasized with asterisks “*”(p < 0.05); “**”(p < 0.01); “***”(p < 0.001) “****”(p < 0.0001). When the differences were non-significant, no identifier was included.
A single administration of VSSP and/or alum as adjuvants for hrEGF-p64 showed no signs of tissue damage at the injection site
Generally observed adverse events associated with different types of Montanide include early injection site reaction; injection site pain and granulomas formation at the injection sites, potentiated by chronic administration, which is imperative to sustain high PAb titers [14]. Thereafter, as a surrogate for local toxicity after vaccination, we performed a histological evaluation of the injection and adjacent sites of immunized mice after a vaccine dose. The animals were sacrificed three days after one immunization and tissue samples were removed from the injection sites (Figure 1).
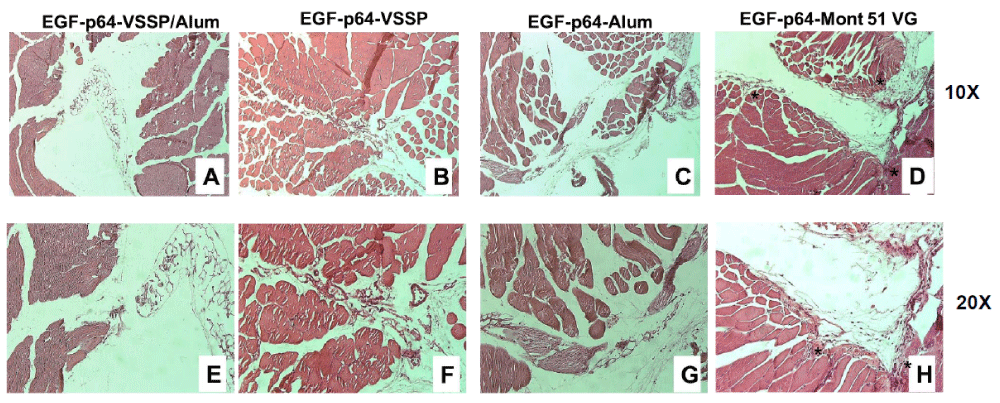
Figure 1: Histopathological evaluation of the muscle tissues from the injection site of mice immunized with EGF-p64 formulated in alternative adjuvants. BALB/c mice received the vaccine formulations in the right flank, intramuscularly (in the case of the formulation adjuvanted in Montanide) or subcutaneously (for the rest of the groups). Normal histological structure of the longitudinal and transverse muscle fibers adjacent to the inoculation site for mice receiving 10 μg equivalent of EGF contained in 63 μg of hrEGF-P64k conjugate adjuvanted in VSSP/Alum (A and E), VSSP (B and F) or Alum (C and G). Injection and adjacent site with cellular infiltrate (lymphocytes and inflammatory) and discontinuity of longitudinal muscle fibers (*) corresponding to the group adyuvated in Mont (D and H). The photographs correspond to one animal per group. H & E, A-D = 10X; E-H = 20X.
Three different lesions were studied: infiltration of lymphocytes, inflammatory infiltrate, and invasion of muscle tissue. None of the groups receiving hrEGF-P64k adjuvanted with VSSP/Alum, VSSP, or Alum showed tissue damage, only the empty space that corresponds to the inoculation site (Figure 1A-C and E-G). However, as expected injection site tissue from mice immunized with Montanide presented all lesions analyzed, Figure 1D,H. In addition, the normal architecture of muscle tissue was lost, Figure 1D,H show the discontinuity of muscle fiber of the adjacent site due to the invasion of fibroblastic reparation tissue. Then, subcutaneous administration of the VSSP/Alum-adjuvanted formulation that doesn´t show signs of local toxicity could enhance the tolerability of the vaccine in the long term.
Maintenance doses in the immunization schedule with hrEGF-P64k adjuvanted in VSSP/Alum are needed to guarantee long-lasting high titers of EGF-specific PAbs
To compare the immunogenicity of the hrEGF-P64k conjugate formulated in VSSP/Alum, different immunization schedules were conducted. As a regularity, BALB/c mice were immunized with 63 µg of the hrEGF-P64k conjugate (equivalent to 10 μg of EGF), using VSSP/Alum or Montanide ISA 51 VG as adjuvants. Though Alum is not a suitable adjuvant for cancer vaccines [15], it was included as single adjuvant control in all the experiments conducted to determine its contribution to the overall response of the combined adjuvant. Unlikely, VSSP is a less potent PAbs inducer in comparison to Alum or Montanide, for which it was not included as single adjuvant control in these protocols (Caballero, et al. 2021). Doses of 200 µg for VSSP and 1 mg for Alum, as well as adsorption conditions used, were based on previous studies [16,17].
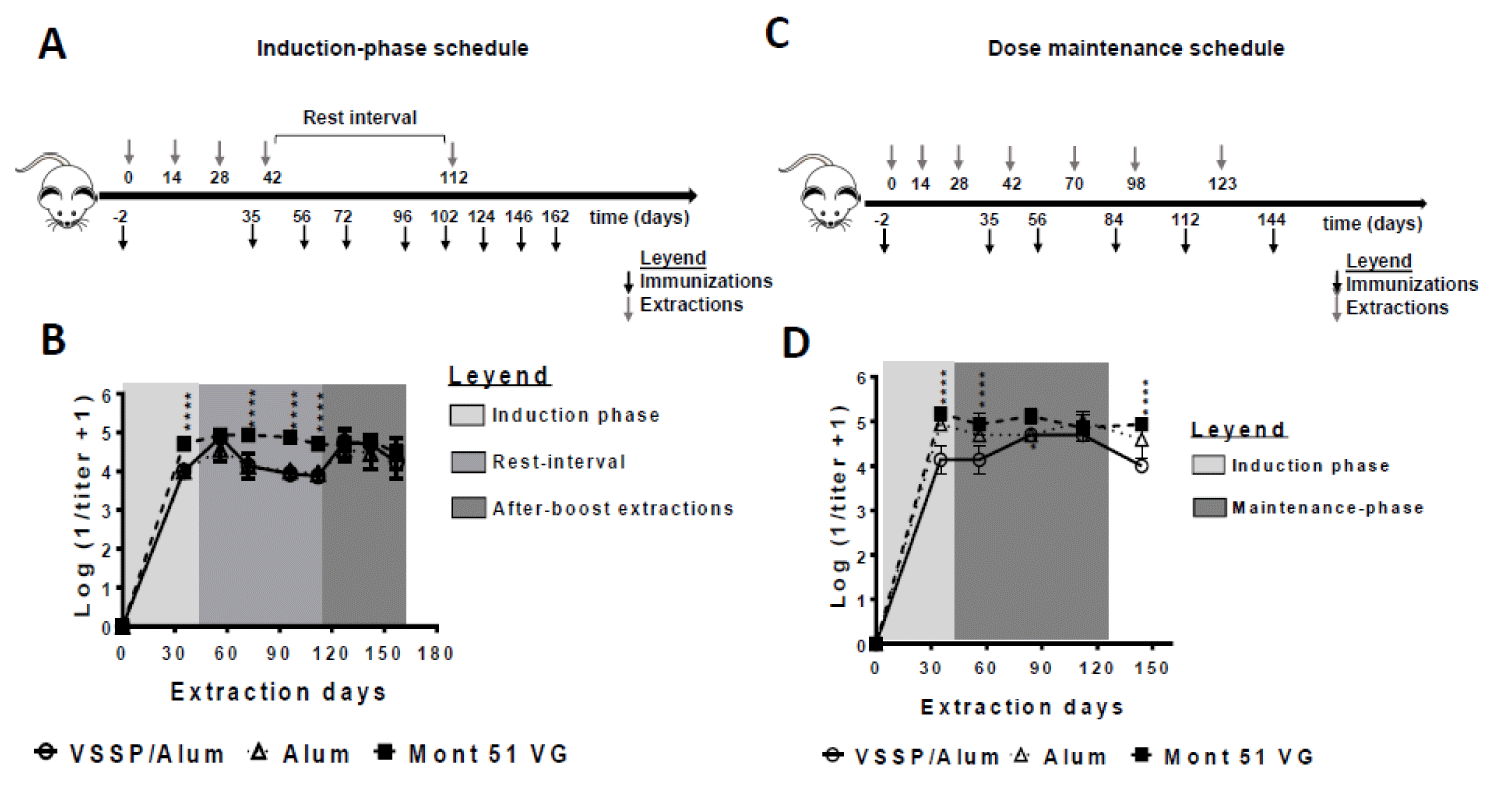
The first experimental schedule consisted of four immunizations spaced biweekly (induction phase) followed by a re-stimulation after three months (Figure 2A). As observed, all evaluated formulations were immunogenic, inducing high titers of EGF-specific PAbs (Figure 2B). In an intermediate extraction (at day 35) Montanide-adjuvanted formulation was superior. However, once all four doses of the induction phase have been administered, titers match, as seen in a further extraction (day 56, second extraction day in Figure 2B). Next, a significant decrease in the titers was observed for the groups that did not receive Montanide one month after the fourth dose (rest interval). However, once mice were boosted with the corresponding vaccine formulations at day 112, the PAbs titers were restored and remained overlapped during the subsequent blood extractions (up to 50 days after the boost). During this time interval, no significant differences were found among PAbs titers for groups receiving VSSP/Alum or control adjuvants (Figure 2B).
Aiming to infer how the PAbs titers induced by this vaccine formulation might behave in the clinic setting, an additional schedule was performed. Such protocol reproduces CIMAvax-EGF’s clinical administration schedule, consisting of four biweekly spaced immunizations (induction phase) and three monthly-spaced re-stimulations (maintenance phase) (Figure 2C). Like in the previous scheme, animals receiving VSSP/Alum achieved lower PAbs titers during the induction phase, where the Montanide-adjuvanted formulation was consistently superior, but this time, titers reached the same order only during the maintenance phase, matching the current formulation after the first boost-dose. (Figure 2D). Also, the persistence of high PAbs titers in this group was dependent on successive maintenance doses, as evidenced by the lower titers obtained on day 144 (three weeks after the last immunization). The need for maintenance doses in the clinical setting to reach the same magnitude for the humoral response is then suggested.
Figure 2: Influence of the immunization schedule on the PAbs titer induced with hrEGF-P64k using different adjuvants. BALB/c mice (n = 5 in each group) were immunized with 10 μg equivalent of EGF contained in 63 μg of the hrEGF-P64k conjugate. Different adjuvants were used: VSSP/Alum (200 µg VSSP/ Alum 1 mg, sc); Alum (Alum 1 mg, sc) and Mont 51 VG (Montanide ISA51 VG, v/v, i.m). A) Four biweekly separated immunizations (induction doses) and a re-stimulation on day 112 were performed. On indicated days blood was collected and processed to obtain serum. B) The scatter plots represent the kinetics for the mean ± SD of the logarithm of the inverse of the IgG isotype EGF-specific antibody titer, determined by ELISA. C) Four biweekly separate immunizations (inductions doses) and three additional doses administered monthly (maintenance doses) were performed. On specified days blood was collected to obtain serum. D) The scatter plots represent the kinetics for the mean ± SD of the logarithm of the inverse of the IgG isotype EGF-specific antibody titer, determined by ELISA. For statistical analysis, a generalized linear model was applied with time and treatments as factors (“*”p < 0.05). The results are representative of two independent experiments for each schedule.
PAbs elicited by the VSSP/Alum adjuvanted-formulation show neutralizing capacity on EGF/EGFR interaction
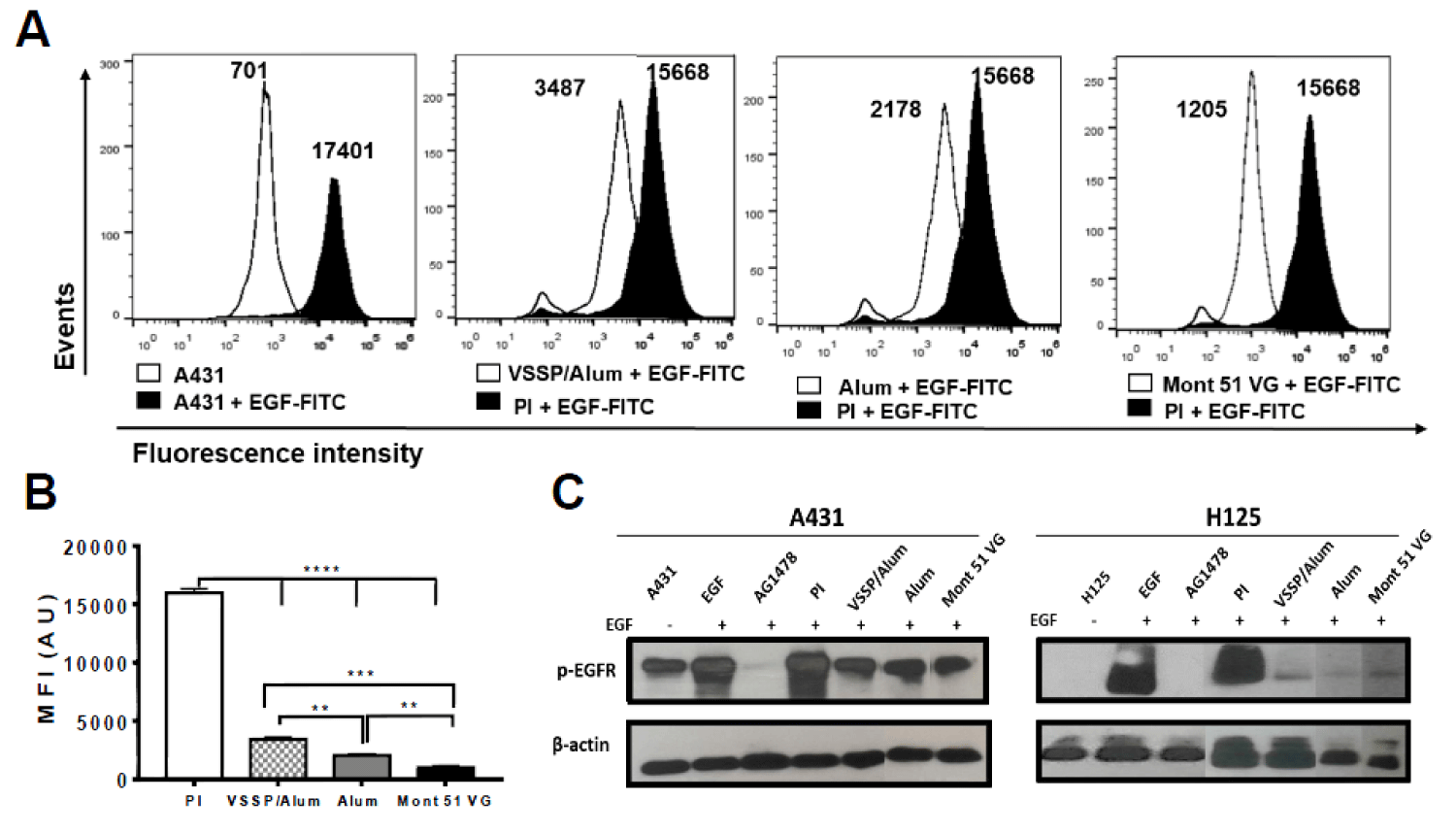
The capability of PAbs contained in the immune sera from immunized mice to prevent EGF binding to its receptor on tumor cells was preliminarily evaluated by flow cytometry. In this assay, A431 cells overexpressing high levels of EGFR (106 molecules/cell) [18], were stained with EGF-FITC pre-incubated with immune serum, or preimmune serum included as negative control (Figure 3A). As observed, a decrease in the mean fluorescence intensity (MFI) was found in the cells where fluorescent EGF was pre-incubated with the PAbs from vaccinated mice, with regard to EGF-FITC pre-incubated with preimmune sera. This behavior was significant for all the groups evaluated, though the magnitude of the effect was higher for mice immunized with the Montanide-adjuvanted formulation (Figure 3B). The lack of inhibitory effect for the preimmune sera suggests the specific association of such inhibition with vaccination-induced PAbs (Figure 3A).
Figure 3: Inhibition of EGF binding by the induced PAbs and its impact on EGFR phosphorylation. A) Pooled sera from each group of mice collected on day 124 of the “induction schedule” protocol (1:100) were incubated with 2 μg/mL of EGF-FITC for 30 min. Next, A431 cells were labeled with this preparation for 20 min. Overlapping histograms show the mean fluorescence intensity (MFI) of unlabeled A431 cells labeled with EGF-FITC in the presence of pre-immune serum (PI) or immune sera from each group. A representative experiment of three conducted is shown. B) The bars represent the mean ± SD of the MIF values obtained for each condition evaluated. Differences among means were analyzed using one-way ANOVA with Dunnett’s post-test. C) Pooled sera from each group collected for day 124 “induction schedule” protocol (1:100) were incubated with 100 ng/mL of EGF for 30 min. Next, A431 or H125 cell lines were stimulated with this preparation for 10 min. Untreated cells and cells incubated with PI serum (1:100) were used as negative controls for EGFR activation, while TKI AG1478 (10 μM) was used as a positive control for EGFR inhibition. Detection of the specified proteins was performed by Western blot. Autoradiographic films correspond with the detection of phosphorylated EGFR in cells (upper panels) or β-actin (lower panels). The results obtained in a representative experiment of two performed for each cell line are shown. In the right image, the samples were re-organized to homogenize samples in order.
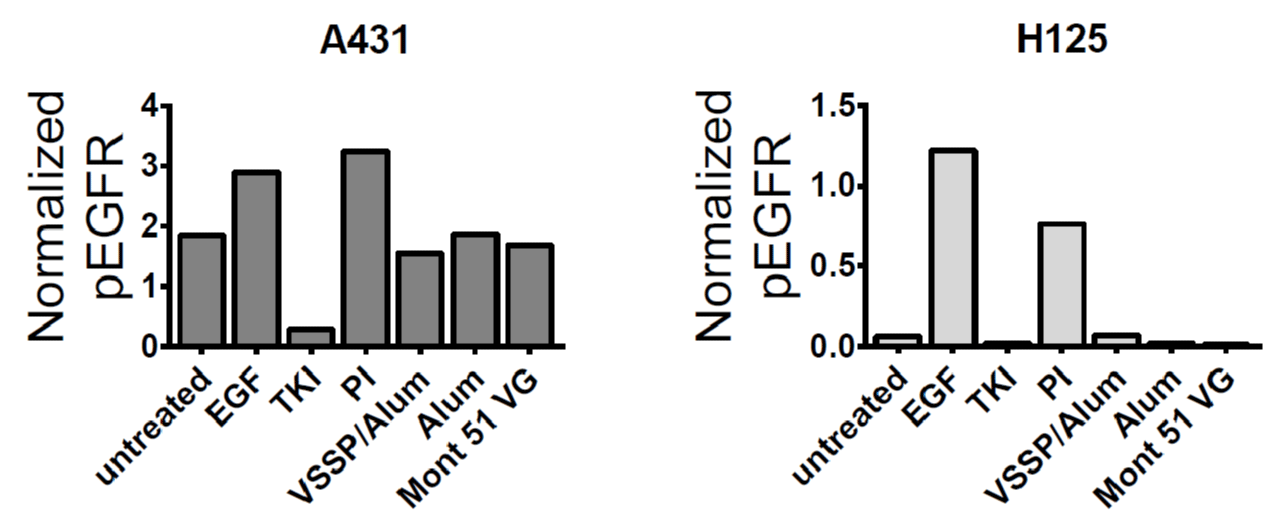
It was also determined how this inhibitory potential impacted the inhibition of EGF-induced EGFR phosphorylation, using A431 and H125 cell lines with differential expression of the receptor as models. In both lines, it was obtained a decrease in the intensity of the bands corresponding to phosphorylated EGFR for the cells treated with immune sera from vaccinated mice with regards to cells incubated with preimmune sera. This effect was more noticeable for H125 cells, whose EGFR expression levels are lower than A431 in an order of magnitude [19] and favors complete neutralization of the receptor (Figure 3C and S1).
Figure S1: Quantification of the inhibitory effect of the PAbs induced by alternative formulations on EGFR phosphorylation. A431 (left graph) or H125 (right graph) cells were pulsed for 10 min at 37 °C with pooled sera from each group collected for day 124 of the “induction schedule” protocol (1:100) pre-incubated for 30 min with 100 ng/mL of EGF. Untreated cells and cells incubated with PI serum (1:100) were used as negative controls for EGFR activation, while TKI AG1478 (10 μM) was used as a positive control for EGFR inhibition. Detection of phosphorylated EGFR (at Y1068 residue) and β-actin was performed by Western blot. In the graphs, columns represent the intensity of the bands corresponding to phosphorylated EGFR in the autoradiographic films normalized considering loading control, quantified using Image J software. These results are representative of two independent experiment conducted for each cell line.
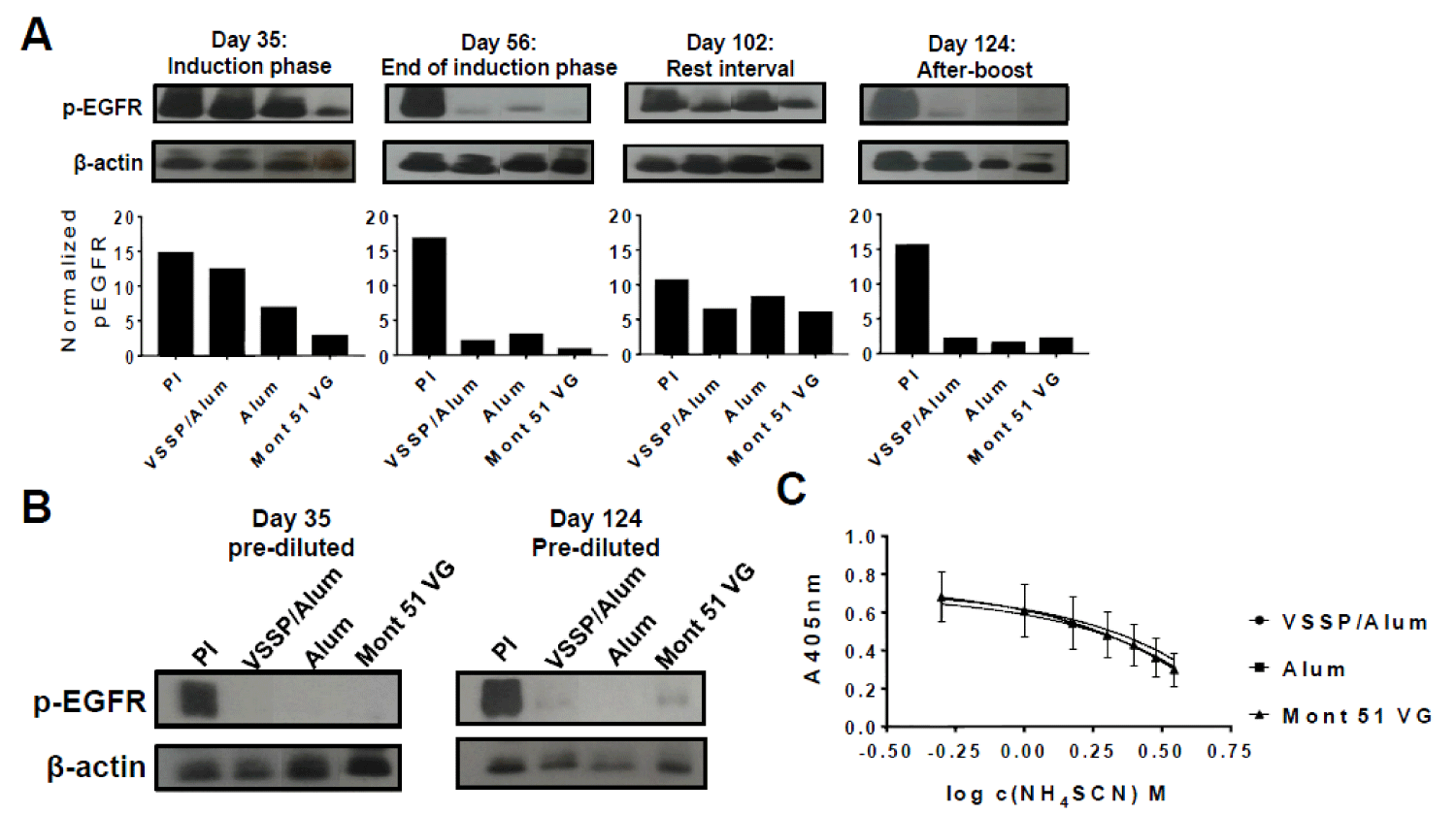
The magnitude of PAbs titers affects the extension of EGF-induced EGFR inhibition, despite similar neutralizing potential
Previous reports have demonstrated an inverse correlation between anti-EGF PAbs titers and EGF-induced EGFR phosphorylation [20]. Then, we decided to evaluate how the magnitude of the PAbs titers induced by the alternative formulations, at different time-points of the “induction-phase schedule” (Figure 2A-B) influenced the inhibition of EGF-mediated receptor phosphorylation (p-EGFR). Thereafter, blockade of EGFR activation by anti-EGF PAbs induced at different extraction days of this schedule was characterized (Figure 4A). Of note, since induced PAbs recognize EGF and not its receptor, a structural constitutive protein (β-actin) was considered as a loading control and used to normalize p-EGFR bands intensity in densitometry analyses. On day 35 (after three doses of the induction phase) inhibition of EGFR phosphorylation was stronger where Montanide was used as an adjuvant, for which PAbs titers were significantly higher (Figure 2B). Next, at the completion of the induction phase, when the titers overlapped, the extent of EGFR phosphorylation inhibition was similar for all the groups. Once immunizations were interrupted (rest-interval) a lower inhibition of ligand-induced phosphorylation of EGFR was observed along with a decrease in the titers of EGF-specific PAbs induced by VSSP/Alum. Finally, an extensive and comparable inhibition of EGFR activation among immunization groups was regained after the boost, consistent with a match in the titers of the PAbs.
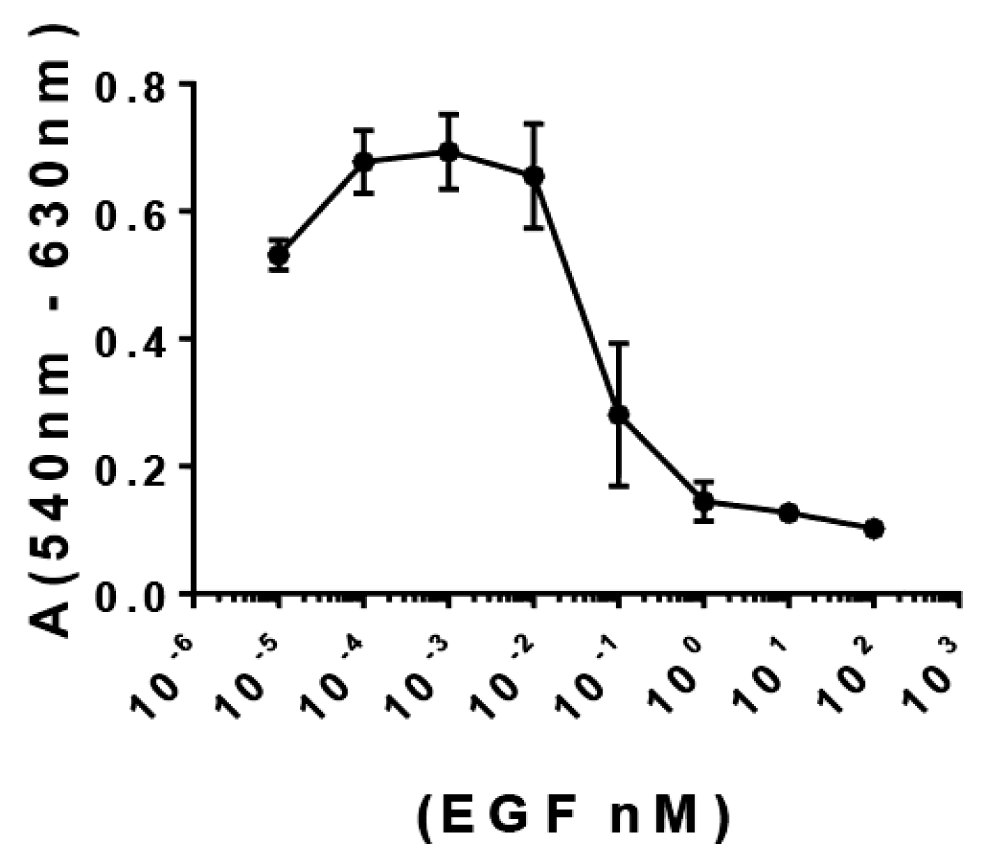
Figure S2: Impact of EGF concentration for A431 cells growth in vitro. A431 cell line (15000 cells/well) were seeded in 96-well plates in DMEM-F12 medium at 10% SFT. Next, the medium was replaced and cells were starved for 16 h. A) Afterwards, cells were incubated for 72 h in DMEM supplemented with increasing concentrations of EGF (ranging from 0-100 nM) and cell viability was evaluated at end-point by MTT. The scatter plot shows the behavior of the difference in the Absorbance at 540 nm and 620 nm (as indicative for cell viability) as function of EGF concentration.
Moreover, when EGF-specific PAbs titers present in the immune sera were pre-diluted and homogenized to 1: 50 000 for all the groups (so the contribution of the observed differences in the titers to the global inhibitory effect was abolished) prior to a further 1:100 dilution and pre-incubation with EGF, inhibition of p-EGFR was extensive and homogeneous among VSSP/Alum-adjuvanted or control groups (Figure 4B). Finally, since the neutralizing potential of the PAbs is related to its binding strength, the avidity of induced PAbs was compared by ELISA. Upon increasing concentrations of a chaotropic agent (NH4SCN) an overlapping decrease in antigen recognition was observed for the PAbs generated by all the formulations evaluated on day 124 of the induction scheme (Figure 4C).
Figure 4: Kinetics inhibition of EGFR phosphorylation and avidity determination. A) H125 cells (105 cells/well) were seeded in 12-well plates and starved for 16 h. Pooled sera from each group (1/100) corresponding to indicated extraction days of the “induction-phase schedule” were incubated with 100ng/mL of EGF for 30 min and cells were stimulated with this preparation for 10 min. Cells incubated with the pre-immune serum PI (1:100) pre-mixed with EGF at the same concentration were included as a specificity control. A) Auto-radiographic films (upper panels) with their corresponding densitometry analysis (lower panels) of phosphorylated EGFR and β-actin levels are shown. In the images, samples were reorganized for homogeneous presentation. B) Alternatively, pooled sera corresponding to indicated days of the “induction-phase schedule” were pre-diluted to homogenize the titers to 1: 50,000, then diluted 1: 100 and incubated with 100 ng/mL of EGF for 30 min. Starved H125 cells were stimulated with this preparation for 10 min. and expression levels of the indicated molecules were analyzed by Western blot. Results of one representative experiment of two performed for each time-point are shown. C) The avidity of EGF-specific antibodies was determined by ELISA in presence of a chaotropic agent. Briefly, antibodies present in the immune serum corresponding to day 124 “induction-phase schedule” was purified by affinity chromatography with protein A and further applied at 100 μg/mL in EGF-coated ELISA plates (10 μg/mL), in presence of increasing concentrations of NH4SCN (ranging from 0 M -4 M). The graph represents the mean ± SD of the absorbance (A) at 405 nm obtained at different NH4SCN concentrations. Samples were analyzed in triplicates. Results of one representative experiment of three performed are shown.
PAbs induced by all the formulations inhibit the viability of A431 cells sensitive to exogenous EGF
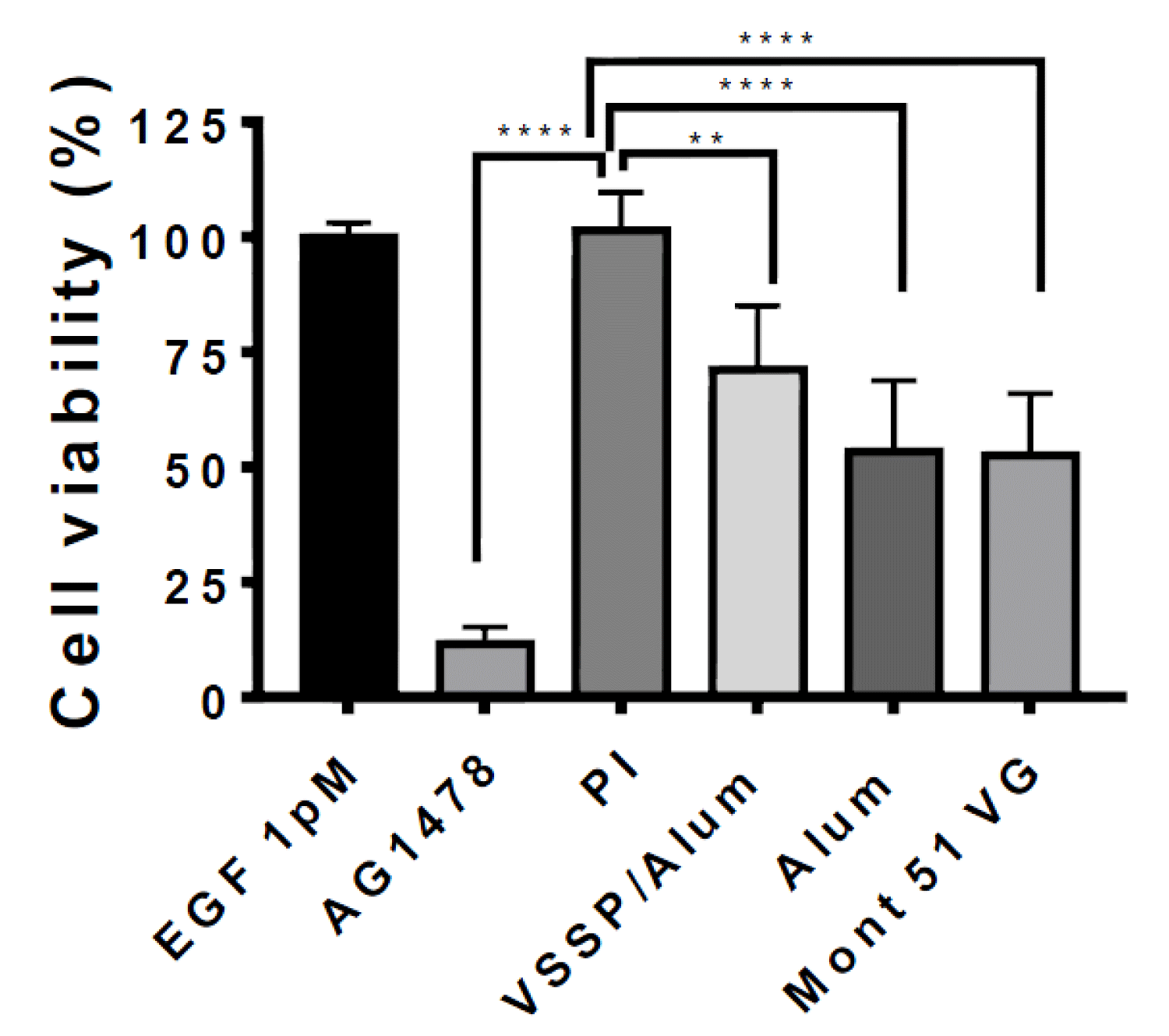
To extend the characterization of the PAbs induced by VSSP/Alum-adjuvanted formulation, their impact on the viability of EGFR-overexpressing A431 tumor cells was evaluated. For this purpose, the sensitivity of A431 cells to increasing concentrations of EGF was initially assessed (Figure S2). Since the higher optical density at 540 nm (proportional to cell viability) was reached by the cells growing in a medium supplemented whit EGF at 1 pm, this condition was selected to further assess the antiproliferative effect of the PAbs. A significant decrease in the cell viability was obtained for the PAbs induced by VSSP/Alum-adjuvanted formulation, when compared to the effect of antibodies contained in the pre-immune serum, for sera obtained at day 140 of the maintenance scheme (Figure 5). Mean cell viability for all immunization groups was lower than the control (preimmune sera) by more than 30%, however, no significant differences were found among VSSP/Alum-adjuvanted or control groups, coincident with the similar titers obtained at this schedule point.
Figure 5: Anti-proliferative effect of the PAbs induced by alternative formulations on EGF-depending A431 cells. A431 cells (15000 cells/well) were seeded in 96-well plates in DMEM-F12 medium at 10% SFT. Next, the medium was replaced and cells were starved for 16 h. Then, cells were incubated for 72 h in DMEM supplemented with 1 pM of EGF in presence of the PAbs (0.25 µg/mL) corresponding to day 140 of the dose maintenance schedule protocol. Cell viability was determined by MTT. PAbs collected from preimmune sera (PI) was used as a negative control and TKI AG1478 (10 μM) was included as a positive control of cytotoxicity. The graph represents the mean ± SD of 6 replicate values, obtained in a representative experiment of three independently conducted. Group means were compared using one-way ANOVA and Dunnett’s post-hoc **(p < 0.01); ****(p < 0.0001).
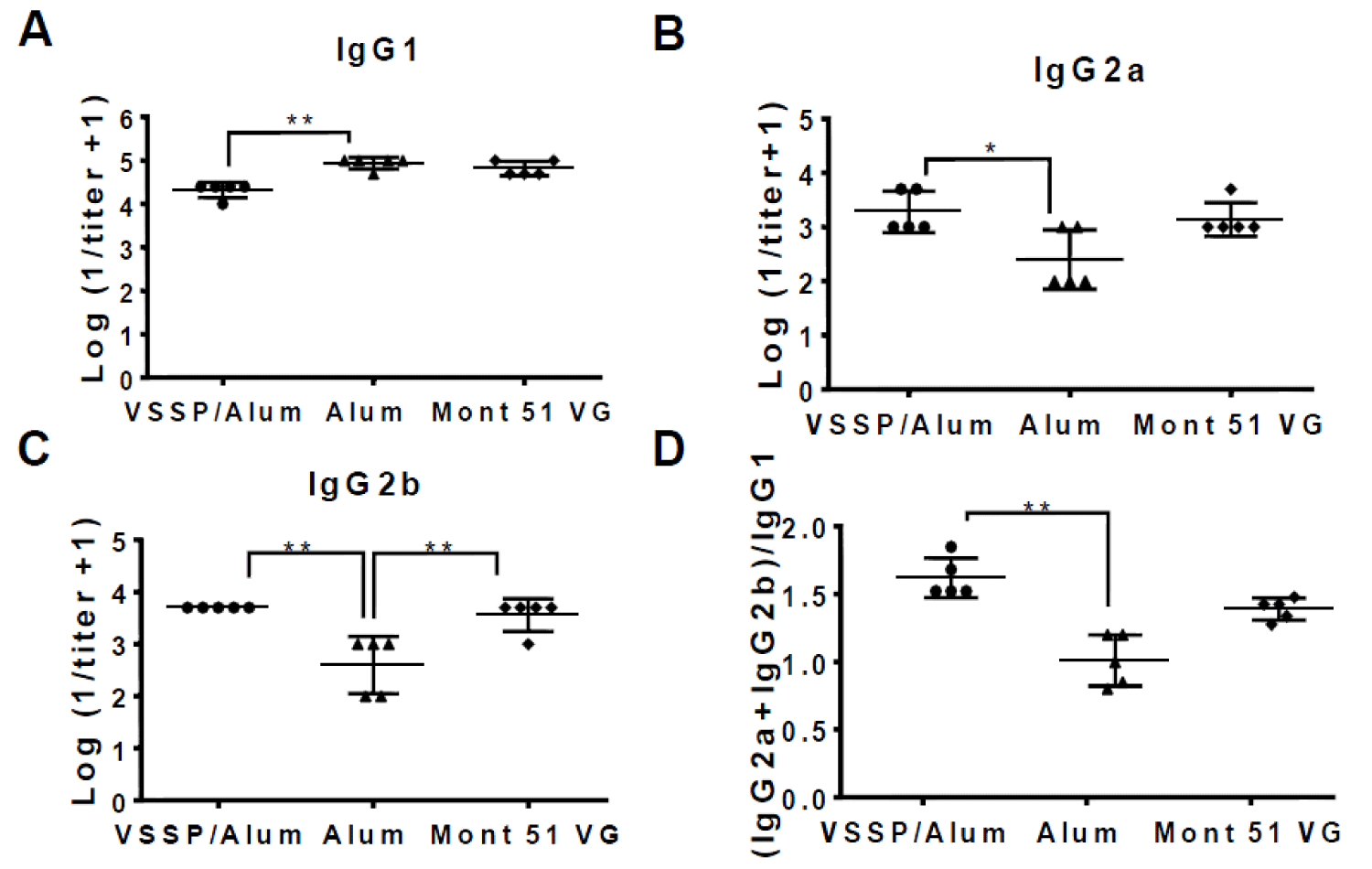
A Th1-like mixed pattern of anti-EGF IgG subclasses is induced by the VSSP/Alum-adjuvanted formulation
IgG subclasses were characterized for EGF-specific PAbs induced in mice receiving alternative formulations above mentioned, for sera obtained after the fourth dose at day 35 of the induction-phase schedule (Figure 6). Formulations including VSSP/Alum or controls induced titers of IgG1, IgG2a, and IgG2b, as detected within the pool of anti-EGF PAbs. However, the titers corresponding to the IgG1 subclass were statistically higher for the group receiving Alum as the only adjuvant, as expected for this Th2-response-inducing adjuvant (Figure 6A). For the IgG2a and IgG2b subclasses, higher and equivalent titers were achieved by the groups adjuvanted in VSSP/Alum or Montanide (Figure 6B,C). When the (IgG2a+IgG2b)/IgG1 ratio was determined, as a surrogate of the Th1/Th2 response pattern, mice immunized with the formulations adjuvanted in VSSP/Alum were significantly higher than the ones receiving Alum as a single adjuvant and similar to Montanide control (Figure 6D).
Figure 6: IgG-specific isotype PAbs subclasses are induced by the alternative formulations. The graphs represent the logarithm of the inverse of the titer of the EGF-specific IgG isotype antibody subclasses A) IgG1 B) IgG2a and C) IgG2b present in the sera from mice immunized with EGF-p64k in VSSP/Alum, Mont 51 VG or Alum control corresponding to day 35, determined by ELISA. D) (IgG2a + IgG2b)/IgG1 ratio is shown for the evaluated formulations. Non-parametric Kruskal-Wallis test, followed by Dunn’s post-hoc test was used to compare group means in the graphs, with exception of IgG2b, for which one-way ANOVA followed by Tukey’s multiple comparisons were applied (“*”p < 0.05; “**”p < 0.01). Two independent experiments were performed for each subclass and representative results are shown.
PAbs are elicited by VSSP/Alum-adjuvanted formula-tions in tumor-bearing or immunosuppressed mice
Once the immunogenicity of hEGF-P64k in the VSSP/Alum formulation was evaluated in healthy mice, in terms of humoral response, specific PAbs induction was also tested in the context of immune suppression, as expected in the scenario of tumor progression. Since previous studies suggested that VSSP could modulate immune regulatory cells and activate dendritic cells [12], in these experiments VSSP was also included as single adjuvant control.
Herein, BALB/c mice were inoculated subcutaneously with F3II mammary tumor cells without expression of autologous EGFR (thus, insensitive to inhibition of the EGF/EGFR axis) and with a demonstrated ability to induce chronic inflammation and immunosuppression [21] Then, mice were further immunized with the indicated formulations (Figure 7). A control group of healthy mice immunized with hr-EGF-p64 adjuvanted in Montanide was included. Considering tumor kinetic, three doses were administered biweekly before animals were sacrificed by tumor burden. Noticeably, EGF-specific PAbs titers induced after three doses were equivalent among formulations containing VSSP/Alum or Montanide in tumor-bearing mice, while no differences in the titers were found between healthy or tumor-bearing mice immunized with Montanide-adjuvanted formulation.
Figure 7: Immunogenicity of hrEGF-P64k in different adjuvants in tumor-bearing mice. A) BALB/c mice (n = 20) were inoculated with 106 cells of the F3II line (s.c). On day 10 the tumor burden was verified and the animals were randomized in four groups (n = 5) and immunized with 10 μg equivalent of EGF adjuvanted with VSSP/Alum (VSSP 200 µg/Alum 1 mg, sc), VSSP (VSSP 200 µg) Alum (Alum 1 mg, sc) or Mont (Montanide ISA 51 VG v/v, im). Immunizations were spaced biweekly on indicated days and the animals were sacrificed when the ethical endpoint was reached. The fifth group of healthy animals (unchallenged with tumor cells) and immunized with 10 μg equivalent of EGF adjuvanted in Mont 51 VG was included as a control. B) Graph represents the mean ± standard deviation (SD) for the logarithm of the titer inverse for anti-EGF PAbs present in the immune serum, determined by ELISA. Differences among groups’ means were analyzed with a non-parametric Kruskall-Wallis test, followed by Dunn’s post-hoc.
Finally, since chemotherapy can also alter the immune response to vaccination [22] we evaluated the induction of EGF-targeting PAbs by the alternative formulations in mice receiving cyclophosphamide (CY, 200 mg/Kg) two days before the start of the immunization schedule. Previous reports refer to a decrease in spleen cellularity and immune cell functionality up to three days after the administration of this chemotherapeutic agent in mice [23]. In this context, the formulations containing VSSP/Alum or Montanide as adjuvants were also able to induce comparable titers of EGF-specific PAbs (Figure S3) and it was observed scorrespondence between these titers and the ones induced in healthy mice, after the same number of immunizations (Figure 2B).
Figure S3: Immunogenicity of hrEGF-P64k in VSSP/Alum or Mont in immunosuppressed mice. A) BALB/c mice (n = 5) were inoculated with 200 mg/kg of cyclophosphamide CY (i.p). On days 0 and 7 mice were with immunized with 10 μg equivalent of EGF adjuvated with VSSP/Alum (VSSP 200 μg/Alum 1 mg, sc) or Mont 51 VG (Montanide ISA 51 VG, v / v, im). B) Graphs represent group means ± standard deviation (SD) of the logarithm of the titer inverse for anti-EGF-specific PAbs present in the immune sera obtained seven days after the second immunization, as determined by ELISA.
EGFR and its ligands had become important therapeutic targets for cancer treatment [24]. Several passive therapies like tyrosine kinase inhibitors, as well as MAbs, have been developed against these targets, some of which are currently registered for the treatment of several malignancies [3]. Nevertheless, cancer vaccines have the potential to endogenously induce an immune response against tumor antigen/s, which constitutes the principle of CIMAvax-EGF. Immunization of cancer patients with this vaccine induces a humoral response against self-EGF and generates PAbs that castrate circulating EGF preventing its interaction with the EGFR expressed on tumor cells, which impairs cancer progression [25]. Remarkable clinical benefit has been demonstrated by CIMAvax-EGF in the treatment of NSCLC upon chronic use [8]. However, the search for innovative alternatives of adjuvant for this vaccine is advisable. Considering the properties of VSSP to polarize the response towards the Th1 pattern and the Alum’s ability to induce high antibody titers, we proposed a combined VSSP/Alum adjuvant for the treatment of cancer patients at advanced stages of the disease, taking EGF as a model for tumor-associated self-antigen. In our study, such combined adjuvant was innocuous at the injection site of immunized mice, while inducing high titers of neutralizing and anti-proliferative PAbs, as desired for an adjuvant chronically administered to cancer patients.
A combination of adjuvants approved for clinical use, with complementary mechanisms of action, has become an attractive alternative in order to potentiate the efficacy of cancer vaccines. In this regard, aluminum salts, phosphate, or hydroxide (Alum), characterized by the induction of strong and long-lasting IgG responses, are the most extensively used adjuvants [26]. However, Alum-induced immune response is polarized towards a Th2 pattern, which is rather undesirable for cancer vaccines, since it might promote, instead of counteracting, tumor progression [15]. On the other hand, VSSP is composed of an outer-vesicle membrane derived from N. meningitides and contains N-acetylated GM3 ganglioside in its structure. Even though PAbs titers generated with VSSP as a single adjuvant are lower compared to other approved adjuvants [27], it behaves as an immune system modulator with a unique ability to simultaneously activate DCs (and polarize the immune response towards a Th1 pattern) and antagonize the functionality of myeloid-derived suppressor cells (MDSC) recruited by the tumor [12,28]. Also, it has been used as an adjuvant in the clinic in combination with other adjuvants, showing a remarkable safety profile [29,30].
The absence of damage in the muscle fibers, as well as the absence of immune, infiltrate in the tissue adjacent to the injection site for mice immunized with the formulation containing VSSP/Alum, suggest its better tolerability than the current adjuvant, which was one of the premises for its proposal as a suitable adjuvant to be used for chronic administration to cancer patients. Whit regard to the induced humoral response, VSSP/Alum combination was able to generate high titers of specific PAbs in BALB/c mice, under different immunization schedules. Nevertheless, maintenance immunizations were required to reach and sustain over time high anti-EGF-PAbs titers induced by the current vaccine formulation, included as a control. The generation of a deposit at the injection site by Montanide adjuvants allows a slower release of the antigen and guarantees the generation of high and long-lasting PAbs titers, but is also the basement of its undesirable side effects [10]. Of note, no significant differences in the level of induced antibodies were found between the groups receiving the VSSP/Alum or Alum as adjuvants, suggesting that the incorporation of VSSP did not affect the ability of Alum to induce high antibody titers [31]. However, the addition of VSSP to Alum did switch the IgG subclasses towards a Th1-pattern. Even though this is a desirable effect for a cancer vaccine adjuvant, this evidence could be complemented with the evaluation of cytokines associated with Th1 or Th2 patterns like IFNγ or IL4, respectively.
Even though an increase in the titers with regard to the current vaccine formulation was not intended, it would be interesting to evaluate an increase in the VSSP dose, based on previous studies that reported an improvement in the titers of PAbs targeting self-antigens when the VSSP dose was increased [32]. Also, increasing the stability and adsorption coefficient of the particle formed by Alum and the antigen could benefit the PAbs response induced, as demonstrated for hepatitis B surface antigen [33].
On the other hand, it has been proposed that the efficacy of CIMAvax-EGF relies on the castration of circulating EGF by the induced PAbs [20]. Blockade of EGF/EGFR interaction by induced PAbs has correlated with greater survival in patients treated with this vaccine [34]. Moreover, the analysis of large clinical trials conducted with this vaccine allowed the establishment of EGF concentration in patient sera as a predictive biomarker of its clinical benefit. Hence, patients, where EGF concentration in sera was above 870 pg/mL, benefited from the administration of the vaccine, in agreement with its major mechanism of action (Rodriguez, et al. 2016). Herein, adjuvant in VSSP/Alum induced PAbs able to prevent EGF binding to its receptor, as well as subsequent phosphorylation at tyrosine 1068, connected to Grb2 adaptor protein and, subsequently, to ERK1/2 MAPK, enrolled in cell proliferation and survival enhancement [35]. Furthermore, when a kinetic characterization of the magnitude of this inhibitory effect was conducted for the “induction-phase scheme”, a nice association was found between specific PAbs titers and the inhibition of EGFR activation. This is, for time-points where VSSP/Alum-induced PAbs titers were inferior to Montanide´s (before completion of the induction phase and during rest-interval) inhibition of p-EGFR was less complete for the combined adjuvant-containing group. However, at time points where PAbs titers coincident for VSSP/Alum and Montanide control group (at the end of induction phase and after boost), similar and extensive inhibition of EGFR activation was achieved. Our results, in agreement with previous clinical evidence, suggest a proportional association between PAbs titers and their ability to inhibit EGFR phosphorylation [20], even though their intrinsic avidity, neutralizing, and anti-proliferative potential are equivalent. Then, optimization of the immunization schedule with hrEGF-P64k adjuvanted in VSSP/Alum in the clinical setting must focus on ensuring the induction of high and sustained PAbs titers in the long term.
Interestingly, immunization of mice compromised by tumor burden or administration of chemotherapy led to the induction of comparable PAbs titers between VSSP/Alum and Montanide adjuvant, suggesting the feasibility of the first to be administered in cancer patients, frequently submitted to extensive chemotherapy regimens. A previous report evidenced that CY-induced immunosuppression increased the response of anti-EGF PAbs and modified immunological dominance in response to CIMAvax-EGF [36]. Furthermore, plasma cells from immunized patients secreting specific PAbs increased after the first administration of the vaccine when combined with CY [37]. This could explain a coincidence in the PAbs titers for VSSP/Alum or Montanide-adjuvanted groups after a few doses, unlike what was obtained in healthy mice. Also, induction of equivalent PAbs titers in tumor-bearing mice for VSSP/Alum or Montanide control was attained. In the context of immunization with CIMAvax-EGF, induction of EGF-specific antibodies in patients immunosuppressed by tumor progression has been previously reported [8]. Also, although F3II tumors are adequate to establish an immunosuppressive environment, this model is negative for autologous EGFR expression, then is not useful to assess the survival of vaccinated mice (and to associate this effect with the induced EGF-specific PAbs response). Since our vaccine formulation is based on human EGF (which is able to bind and activate both human and mice EGFR) to evaluate the impact on the survival of immunized tumor-bearing mice, it would be possible to challenge immunized mice with 3LL Lewis lung carcinoma, that overexpress murine EGFR [38], or some murine tumor model modified to stably express heterologous human EGFR (for instance, through lentiviral transduction). In this scenario, human recombinant EGF could be administered in a controlled manner to the mice, so the antitumor effect observed could be associated with the castration of the administered hrEGF by the induced PAbs.
Finally, despite the long-term survival has been demonstrated for cancer patients immunized with CIMAvax-EGF [39] patient relapse associated with the emergence of tumor-resistant variants could be expected, as observed for different EGFR-targeting therapies [40]. The most common resistance-driving mutation in response to EGFR-targeting TKIs is a threonine–methionine amino acid substitution at position 790 (T790 M) of HER1 [3]. Also, HER1-exon 19 deletions are the most recurrent activating mutations in advanced non–small cell lung cancer (NSCLC) [41]. In contrast, resistance to MAbs is often associated with bypass signaling driven by genomic alterations in downstream signaling molecules like KRAS which activate downstream pathways ERK1/2 [42]. Compensatory up-regulation of additional HER family members like HER2, HER3, or related receptors like Axl and MET in response to chronic treatment with cetuximab enables bypass signaling and tumor recurrence [43]. Finally, alterations in the antigen-presentation machinery of tumor cells, and overexpression of checkpoint molecules as PD-L1 could compromise the benefit of this vaccine [44]. It would be of interest to characterize the efficacy of EGF neutralization by the induced PAbs in tumor models representative of these resistance mechanisms.
In summary, immunization of BALB/c mice with hrEGF-p64 adjuvanted in VSSP/Alum generates high titer of PAbs with comparable intrinsic avidity and neutralizing potential than those induced by the current vaccine formulation. The potentialities of VSSP/Alum combination in cancer immunotherapy as a well-tolerated and immunogenic adjuvant are, then, suggested. Also, the use of the VSSP/Alum combined adjuvant might incorporate novel properties related to VSSP, like its ability to impair MDSC’s suppressive capacity and prevent their migration to the tumor microenvironment, which could be addressed in future preclinical studies [12]. The safety of this alternative formulation must be first confirmed in non-human primates, while its benefits for the treatment of cancer patients should be then confirmed in the clinical setting in order to assess its efficacy to achieve long-term control of tumor burden, as demonstrated for CIMAvax-EGF.
We acknowledge Gustavo González Ruiz for supplying hrEGF-p64 and expert opinions, as well as Geidy Diana Diaz and Lisandra Padron for excellent technical assistance. The authors are extremely thankful to Dr. Danay Saavedra for her valuable revision of the manuscript.
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012 Jul 12;12(8):553-63. doi: 10.1038/nrc3309. PMID: 22785351.
- Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012 Jan;16(1):15-31. doi: 10.1517/14728222.2011.648617. Epub 2012 Jan 12. PMID: 22239438; PMCID: PMC3291787.
- Uribe ML, Marrocco I, Yarden Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers (Basel). 2021 Jun 1;13(11):2748. doi: 10.3390/cancers13112748. PMID: 34206026; PMCID: PMC8197917.
- Purba ER, Saita EI, Maruyama IN. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The "Rotation Model". Cells. 2017 Jun 2;6(2):13. doi: 10.3390/cells6020013. PMID: 28574446; PMCID: PMC5492017.
- Kjær IM, Olsen DA, Brandslund I, Bechmann T, Jakobsen EH, Bogh SB, Madsen JS. Prognostic impact of serum levels of EGFR and EGFR ligands in early-stage breast cancer. Sci Rep. 2020 Oct 6;10(1):16558. doi: 10.1038/s41598-020-72944-1. PMID: 33024132; PMCID: PMC7538553.
- Bergado Báez G, Hernández Fernández DR, Mazorra Herrera Z, Sánchez Ramírez B. HER1-based vaccine: Simultaneous activation of humoral and cellular immune response. Semin Oncol. 2018 Jan;45(1-2):75-83. doi: 10.1053/j.seminoncol.2018.05.002. Epub 2018 May 31. PMID: 30318087.
- Mancebo Rodríguez A, Bergado Báez G, Acosta Lago E, León Goñi A, Blanco Gámez D, Fuentes Morales D, Hernández Fernández DR, Sánchez Ramírez B, Pérez Barreda A, Casacó Parada Á. Immuno-toxicological evaluation of her1 cancer vaccine in non-human primates: a 6-month subcutaneous study. Immunopharmacol Immunotoxicol. 2021 Jun;43(3):283-290. doi: 10.1080/08923973.2021.1900232. Epub 2021 Mar 16. PMID: 33722157.
- Saavedra D, Crombet T. CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients. Front Immunol. 2017 Mar 13;8:269. doi: 10.3389/fimmu.2017.00269. PMID: 28348561; PMCID: PMC5346887.
- Bowen WS, Svrivastava AK, Batra L, Barsoumian H, Shirwan H. Current challenges for cancer vaccine adjuvant development. Expert Rev Vaccines. 2018 Mar;17(3):207-215. doi: 10.1080/14760584.2018.1434000. Epub 2018 Feb 8. PMID: 29372660; PMCID: PMC6093214.
- Ascarateil S, Dupuis L. Surfactants in vaccine adjuvants: description and perspectives. Vaccine. 2006 Apr 12;24 Suppl 2:S2-83-5. doi: 10.1016/j.vaccine.2005.01.134. PMID: 16823939.
- Oliver L, Fernández A, Raymond J, López-Requena A, Fernández LE, Mesa C. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for antigen-specific cytotoxic T lymphocyte response stimulation under leukopenic conditions. Vaccine. 2012 Apr 19;30(19):2963-72. doi: 10.1016/j.vaccine.2012.02.054. Epub 2012 Mar 3. PMID: 22391399.
- Fernández A, Oliver L, Alvarez R, Hernández A, Raymond J, Fernández LE, Mesa C. Very small size proteoliposomes abrogate cross-presentation of tumor antigens by myeloid-derived suppressor cells and induce their differentiation to dendritic cells. J Immunother Cancer. 2014 Mar 11;2:5. doi: 10.1186/2051-1426-2-5. PMID: 24829762; PMCID: PMC4019907.
- Mancebo A, Casacó A, González B, Ledón N, Sorlozabal J, León A, Gómez D, González Y, Bada AM, González C, Arteaga ME, Ramírez H, Fuentes D. Repeated dose intramuscular injection of the CIMAvax-EGF vaccine in Sprague Dawley rats induces local and systemic toxicity. Vaccine. 2012 May 9;30(22):3329-38. doi: 10.1016/j.vaccine.2012.01.092. Epub 2012 Mar 17. PMID: 22433960.
- van Doorn E, Liu H, Huckriede A, Hak E. Safety and tolerability evaluation of the use of Montanide ISA™51 as vaccine adjuvant: A systematic review. Hum Vaccin Immunother. 2016;12(1):159-69. doi: 10.1080/21645515.2015.1071455. Epub 2015 Sep 17. PMID: 26378866; PMCID: PMC4962750.
- Gouttefangeas C, Rammensee HG. Personalized cancer vaccines: adjuvants are important, too. Cancer Immunol Immunother. 2018 Dec;67(12):1911-1918. doi: 10.1007/s00262-018-2158-4. Epub 2018 Apr 11. PMID: 29644387.
- Ramírez BS, Pestana ES, Hidalgo GG, García TH, Rodríguez RP, Ullrich A, Férnandez LE. Active antimetastatic immunotherapy in Lewis lung carcinoma with self EGFR extracellular domain protein in VSSP adjuvant. Int J Cancer. 2006 Nov 1;119(9):2190-9. doi: 10.1002/ijc.22085. PMID: 16841332.
- Routhu NK, Cheedarla N, Bollimpelli VS, Gangadhara S, Edara VV, Lai L, Sahoo A, Shiferaw A, Styles TM, Floyd K, Fischinger S, Atyeo C, Shin SA, Gumber S, Kirejczyk S, Dinnon KH 3rd, Shi PY, Menachery VD, Tomai M, Fox CB, Alter G, Vanderford TH, Gralinski L, Suthar MS, Amara RR. SARS-CoV-2 RBD trimer protein adjuvanted with Alum-3M-052 protects from SARS-CoV-2 infection and immune pathology in the lung. Nat Commun. 2021 Jun 11;12(1):3587. doi: 10.1038/s41467-021-23942-y. PMID: 34117252; PMCID: PMC8196016.
- Gong H, Kovar J, Little G, Chen H, Olive DM. In vivo imaging of xenograft tumors using an epidermal growth factor receptor-specific affibody molecule labeled with a near-infrared fluorophore. Neoplasia. 2010 Feb;12(2):139-49. doi: 10.1593/neo.91446. PMID: 20126472; PMCID: PMC2814352.
- Garrido G, Tikhomirov IA, Rabasa A, Yang E, Gracia E, Iznaga N, Fernandez LE, Crombet, R.S. Kerbel T, Perez R. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther. 2011; 11:373-382. doi14097 .
- Popa X, García B, Fuentes KP, Huerta V, Alvarez K, Viada CE, Neninger E, Rodríguez PC, González Z, González A, Crombet T, Mazorra Z. Anti-EGF antibodies as surrogate biomarkers of clinical efficacy in stage IIIB/IV non-small-cell lung cancer patients treated with an optimized CIMAvax-EGF vaccination schedule. Oncoimmunology. 2020 May 25;9(1):1762465. doi: 10.1080/2162402X.2020.1762465. PMID: 32923124; PMCID: PMC7458606.
- Fuentes D, Cabezas-Cruz A, Mesa C, Carmenate T, Martínez D, Valdés-Zayas A, Montero E, Pérez R. Murine Mammary Carcinoma Induces Chronic Systemic Inflammation and Immunosuppression in BALB/c Mice. J Breast Cancer. 2022 Jun;25(3):218-232. doi: 10.4048/jbc.2022.25.e18. Epub 2022 Apr 26. PMID: 35657001; PMCID: PMC9250876.
- Reinhardt D, Houliara K, Pekrun A, Lakomek M, Krone B. Impact of conventional chemotherapy on levels of antibodies against vaccine-preventable diseases in children treated for cancer. Scand J Infect Dis. 2003;35(11-12):851-7. doi: 10.1080/00365540310016600. PMID: 14723361.
- Wang Y, Meng Q, Qiao H, Jiang H, Sun X. Role of the spleen in cyclophosphamide-induced hematosuppression and extramedullary hematopoiesis in mice. Arch Med Res. 2009 May;40(4):249-55. doi: 10.1016/j.arcmed.2009.04.003. Epub 2009 Jun 4. Erratum in: Arch Med Res. 2010 Jan;41(1):66. PMID: 19608013.
- Esparís-Ogando A, Montero JC, Arribas J, Ocaña A, Pandiella A. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr Pharm Des. 2016;22(39):5887-5898. doi: 10.2174/1381612822666160715132233. PMID: 27426127.
- Rodríguez PC, Rodríguez G, González G, Lage A. Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small-cell lung cancer therapy. MEDICC Rev. 2010 Winter;12(1):17-23. doi: 10.37757/MR2010.V12.N1.4. PMID: 20387330.
- HogenEsch H, O'Hagan DT, Fox CB. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines. 2018 Oct 10;3:51. doi: 10.1038/s41541-018-0089-x. PMID: 30323958; PMCID: PMC6180056.
- Morera Y, Bequet-Romero M, Ayala M, Lamdán H, Agger EM, Andersen P, Gavilondo JV. Anti-tumoral effect of active immunotherapy in C57BL/6 mice using a recombinant human VEGF protein as antigen and three chemically unrelated adjuvants. Angiogenesis. 2008;11(4):381-93. doi: 10.1007/s10456-008-9121-5. Epub 2008 Nov 26. PMID: 19034678.
- Mesa C, De León J, Rigley K, Fernández LE. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for Th1 induction and dendritic cell activation. Vaccine. 2004 Aug 13;22(23-24):3045-52. doi: 10.1016/j.vaccine.2004.02.010. PMID: 15297054.
- Caballero I, Aira LE, Lavastida A, Popa X, Rivero J, González J, Mesa M, González N, Coba K, Lorenzo-Luaces P, Wilkinson B, Santiesteban Y, Santiesteban Y, Troche M, Suarez E, Crombet T, Sánchez B, Casacó A, Macías A, Mazorra Z. Safety and Immunogenicity of a Human Epidermal Growth Factor Receptor 1 (HER1)-Based Vaccine in Prostate Castration-Resistant Carcinoma Patients: A Dose-Escalation Phase I Study Trial. Front Pharmacol. 2017 May 10;8:263. doi: 10.3389/fphar.2017.00263. PMID: 28539888; PMCID: PMC5423955.
- Morera-Díaz Y, Gavilondo JV, Bequet-Romero M, Sánchez Ramírez J, Hernández-Bernal F, Selman-Housein KH, Perez L, Ayala-Ávila M. Specific active immunotherapy with the HEBERSaVax VEGF-based cancer vaccine: From bench to bedside. Semin Oncol. 2018 Jan;45(1-2):68-74. doi: 10.1053/j.seminoncol.2018.03.004. Epub 2018 Mar 23. PMID: 30318086.
- Suárez NG, Báez GB, Rodríguez MC, Pérez AG, García LC, Hernández Fernández DR, Pous JR, Ramírez BS. Anti-proliferative and pro-apoptotic effects induced by simultaneous inactivation of HER1 and HER2 through endogenous polyclonal antibodies. Oncotarget. 2017 Aug 3;8(47):82872-82884. doi: 10.18632/oncotarget.19958. PMID: 29137309; PMCID: PMC5669935.
- Morera Y, Sánchez J, Bequet-Romero M, Selman-Housein KH, de la Torre A, Hernández-Bernal F, Martín Y, Garabito A, Piñero J, Bermúdez C, de la Torre J, Ayala M, Gavilondo JV. Specific humoral and cellular immune responses in cancer patients undergoing chronic immunization with a VEGF-based therapeutic vaccine. Vaccine. 2017 Jun 16;35(28):3582-3590. doi: 10.1016/j.vaccine.2017.05.020. Epub 2017 May 20. PMID: 28536029.
- Hansen B, Belfast M, Soung G, Song L, Egan PM, Capen R, Hogenesch H, Mancinelli R, Hem SL. Effect of the strength of adsorption of hepatitis B surface antigen to aluminum hydroxide adjuvant on the immune response. Vaccine. 2009 Feb 5;27(6):888-92. doi: 10.1016/j.vaccine.2008.11.078. Epub 2008 Dec 9. PMID: 19071182.
- García B, Neninger E, de la Torre A, Leonard I, Martínez R, Viada C, González G, Mazorra Z, Lage A, Crombet T. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res. 2008 Feb 1;14(3):840-6. doi: 10.1158/1078-0432.CCR-07-1050. PMID: 18245547.
- Saito T, Okada S, Ohshima K, Yamada E, Sato M, Uehara Y, Shimizu H, Pessin JE, Mori M. Differential activation of epidermal growth factor (EGF) receptor downstream signaling pathways by betacellulin and EGF. Endocrinology. 2004 Sep;145(9):4232-43. doi: 10.1210/en.2004-0401. Epub 2004 Jun 10. PMID: 15192046.
- Gonzalez G, Montero E, Leon K, Cohen IR, Lage A. Autoimmunization to epidermal growth factor, a component of the immunological homunculus. Autoimmun Rev. 2002 Feb;1(1-2):89-95. doi: 10.1016/s1568-9972(01)00015-5. PMID: 12849064.
- Rodriguez PC, Gonzalez I, Gonzalez A, Avellanet J, Lopez A, Perez R, Lage A, Montero E. Priming and boosting determinants on the antibody response to an Epidermal Growth Factor-based cancer vaccine. Vaccine. 2008 Aug 26;26(36):4647-54. doi: 10.1016/j.vaccine.2008.07.003. Epub 2008 Jul 18. PMID: 18640164.
- Garrido G, Rabasa A, Garrido C, López A, Chao L, García-Lora AM, Garrido F, Fernández LE, Sánchez B. Preclinical modeling of EGFR-specific antibody resistance: oncogenic and immune-associated escape mechanisms. Oncogene. 2014 Jun 12;33(24):3129-39. doi: 10.1038/onc.2013.288. Epub 2013 Aug 26. PMID: 23975426.
- Saavedra D, Neninger E, Rodriguez C, Viada C, Mazorra Z, Lage A, Crombet T. CIMAvax-EGF: Toward long-term survival of advanced NSCLC. Semin Oncol. 2018 Jan;45(1-2):34-40. doi: 10.1053/j.seminoncol.2018.04.009. Epub 2018 May 1. PMID: 30318082.
- Wang Q, Greene MI. Mechanisms of resistance to ErbB-targeted cancer therapeutics. J Clin Invest. 2008 Jul;118(7):2389-92. doi: 10.1172/JCI36260. PMID: 18568082; PMCID: PMC2430503.
- Xu CW, Lei L, Wang WX, Lin L, Zhu YC, Wang H, Miao LY, Wang LP, Zhuang W, Fang MY, Lv TF, Song Y. Molecular Characteristics and Clinical Outcomes of EGFR Exon 19 C-Helix Deletion in Non-Small Cell Lung Cancer and Response to EGFR TKIs. Transl Oncol. 2020 Sep;13(9):100791. doi: 10.1016/j.tranon.2020.100791. Epub 2020 May 31. PMID: 32492620; PMCID: PMC7264750.
- Lin L, Bivona TG. Mechanisms of Resistance to Epidermal Growth Factor Receptor Inhibitors and Novel Therapeutic Strategies to Overcome Resistance in NSCLC Patients. Chemother Res Pract. 2012;2012:817297. doi: 10.1155/2012/817297. Epub 2012 Aug 29. PMID: 22970367; PMCID: PMC3437267.
- Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008 Jun 26;27(28):3944-56. doi: 10.1038/onc.2008.19. Epub 2008 Feb 25. PMID: 18297114; PMCID: PMC2903615.
- Santaniello A, Napolitano F, Servetto A, De Placido P, Silvestris N, Bianco C, Formisano L, Bianco R. Tumour Microenvironment and Immune Evasion in EGFR Addicted NSCLC: Hurdles and Possibilities. Cancers (Basel). 2019 Sep 24;11(10):1419. doi: 10.3390/cancers11101419. PMID: 31554160; PMCID: PMC6826622