More Information
Submitted: July 13, 2022 | Approved: July 23, 2022 | Published: July 25, 2022
How to cite this article: Gansauge F, Poch B. Evaluation of long antigen exposition dendritic cell therapy (LANEX-DC®) in the adjuvant treatment of pancreatic cancer – results of a single center analysis. Arch Cancer Sci Ther. 2022; 6: 006-008.
DOI: 10.29328/journal.acst.1001027
Copyright License: © 2022 Gansauge F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pancreatic cancer; Dendritic cells; LANEX-DC®; Immunotherapy; Adjuvant
Abbreviations: DFS: Disease-Free Survival; OS: Overall Survival; LANEX-DC: Long Antigen Exposition Dendritic Cells
Evaluation of long antigen exposition dendritic cell therapy (LANEX-DC®) in the adjuvant treatment of pancreatic cancer – results of a single center analysis
Frank Gansauge1-3* and Bertram Poch1,2
1Center for Oncologic, Endocrine and Minimal-Invasive Surgery, Silcherstr. 36, 89231 Neu-Ulm, Germany
2Shanxi University, Taiyuan, China
3LDG, laboratories Dr. Gansauge, GmbH, Sedanstr. 14, 89077 Ulm, Germany
*Address for Correspondence: Frank Gansauge, Center for Oncologic, Endocrine and Minimal-Invasive Surgery, Silcherstr. 36, 89231 Neu-Ulm, Germany, Email: [email protected]
Introduction: Even after surgical resection and adjuvant chemotherapy in pancreatic cancer the 5-year disease-free survival times (DFS), as well as overall survival rates (OS), are still low and median survival times are below 2 years. Here we retrospectively analyzed the outcome of immunotherapy in the additional adjuvant treatment of pancreatic cancer with long antigen exposition dendritic cell therapy (LANEX-DC®) in 28 patients who were treated at our institution.
Patients: Data were available from 28 patients. Dendritic cells (LAEX-DC®) were produced according to a recently published protocol.
Results: Therapy was well tolerated and no serious side effects were observed. The median disease-free survival times and the median survival times were 16,9 months and 29,4 months respectively. Five-year DFS and OS were 14,3% and 17,9%.
Conclusion: We were able to show in a small cohort of patients that additional treatment with dendritic cells (LANEX-DC®) is highly effective and extends the median disease-free survival times as well as the median survival in the adjuvant treatment of pancreatic cancer, whereas the five-year overall survival still remains unsatisfactory.
Pancreatic cancer has a poor survival rate of 9% [1]. Even after adjuvant chemotherapy following upfront resection of the tumor, approximately 75% of the patients develop disease recurrence within 2 years [2]. Several randomized trials have shown, that adjuvant chemotherapy improves survival in patients after resection of pancreatic cancer [3,4]. By this adjuvant chemotherapeutic regimen using gemcitabine the median disease-free survival times (DFS) were increased to 13,4 months and the median overall survival times (OS) were increased to 22,8 months as compared to an OS of 20,2 months in the observation group [3].
Dendritic cells (DC) are the most potent antigen-presenting cells in the body, presenting tumor antigens to T lymphocytes and inducing an anti-tumor immune response [5]. Several studies have indicated that dendritic cell therapy is effective in the palliative treatment of different types of cancer [6]. In small series, it has been indicated that adjuvant dendritic cell therapy following curative resection for ductal adenocarcinoma of the pancreas might improve DFS and OS [7,8]. We have recently reported on the beneficial effects of dendritic cell therapy using DC in the additional palliative treatment of patients suffering from pancreatic cancer [9] and the adjuvant treatment in patients with gastric cancer [10]. In the following retrospective analysis, we investigated the outcome of adjuvant immunotherapy with DC (LANEX-DC® - long antigen exposition dendritic cells) in patients following surgical resection for pancreatic cancer.
28 patients suffering from pancreatic carcinoma have been vaccinated with autologous dendritic cells at our institution. Follow-up data of all patients were available. All patients alive were observed for at least 5 years. The mean age was 62,7 years (31,5 to 77,5 years). Thirteen patients were male, fifteen patients were female. Histopathological examination revealed in all patients adenocarcinoma of the pancreas. In ten patients a pancreatic left resection and in 18 patients a pancreatic head resection was performed. In 24 patients an R0-resection was possible whereas 4 patients were R1 resected (14,3%). With regard to staging 2 patients showed UICC stage Ia, 6 patients stage Ib, 5 patients stage IIa, 11 patients stage IIb and 4 patients stage III. 20 Patients underwent adjuvant chemotherapy according to the guidelines (mainly 5-FU or gemcitabine chemotherapy). Immunotherapy with DC was carried out in a mean of 21 days following surgery.
All of the patients gave written informed consent for additional treatment with LANEX-DC®.
Generation of mature antigen-loaded monocyte-derived dendritic cells: The whole procedure for gaining the mature dendritic cells was performed according to Good Manufacturing Practice standards (Certificate of GMP compliance DE_BW_01_GMP_2021_0171). Serum-pulsed LANEX-DC® (long antigen exposition dendritic cells) were produced as described recently [10]:
Peripheral blood mononuclear cells (PBMCs) were isolated from 200 ml of heparinized venous blood of the patient by density gradient centrifugation (Biocoll®, Biochrom, Germany). PBMCs were seeded in 6-well-plates (BD Falcon, Heidelberg, Germany), and after 2 hours the non-adherent cells were removed. Adherent cells were cultured in RPMI 1640 (Sigma-Aldrich, Munich, Germany) supplemented with 10% of the patient’s serum and 2 mM L-glutamine (all Sigma-Aldrich, Munich, Germany) in the presence of 750 U/ml rh-GM-CSF and 500 U/ml rh-IL-4 (both CellGenix, Freiburg, Germany) for 7 days. On day 4, media was removed and non-adherent cells were collected from the old media by centrifugation and resuspended in fresh RPMI 1640 supplemented with 10% serum. Again, 750 U/ml rh-GM-CSF and 500 U/ml rh-IL-4 were added. Maturation of moDCs was induced by adding 20 ng/ml rh-IL-1β, 20 ng/ml rh-TNF-α and 60 ng/ml rh-IL-6 (all CellGenix, Freiburg, Germany). After 24 hours, moDCs were harvested, washed twice in sterile PBS, and an aliquot of the cells was removed for phenotypic analysis and sterility testing. moDCs for immediate vaccination (day 7 of treatment protocol) were resuspended in 1ml sterile saline solution containing 10% autologous serum and administered by intradermal injection in the abdominal cutis. To avoid loss of activity by freezing/thawing the DC was always given directly after production was completed.
Statistical analysis
Kaplan-Meier estimates were computed using MedCalc® software.
A total of 28 patients were treated with LANEX-DC® postoperatively following curative resection for pancreatic cancer. Except for light flu-like symptoms on the day of reinjection of the dendritic cells in 6 patients (WHO II, 21,4%), no serious side effects were observed. During the 5-year observation period, 24 patients developed relapse of pancreatic cancer (1 patient with bone metastases, 1 patient with pulmonary metastases, 8 patients with liver metastases, and 14 patients with local relapse/peritoneal metastases). The median time for recurrence in these 24 patients was 15,6 months ranging from 3,7 to 51,5 months.
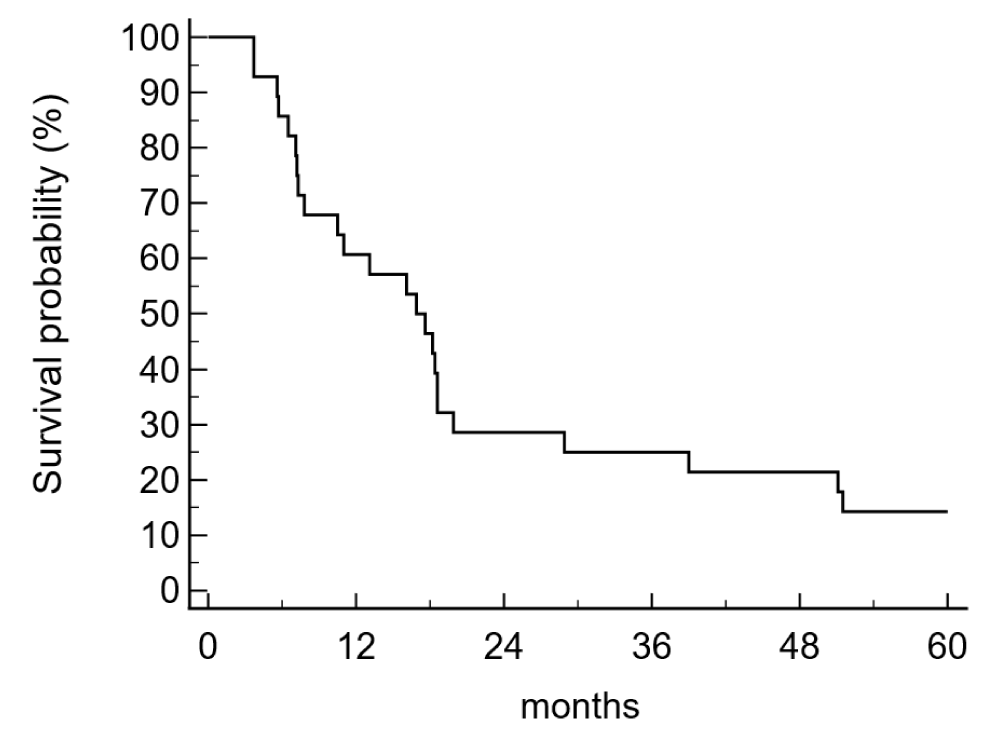
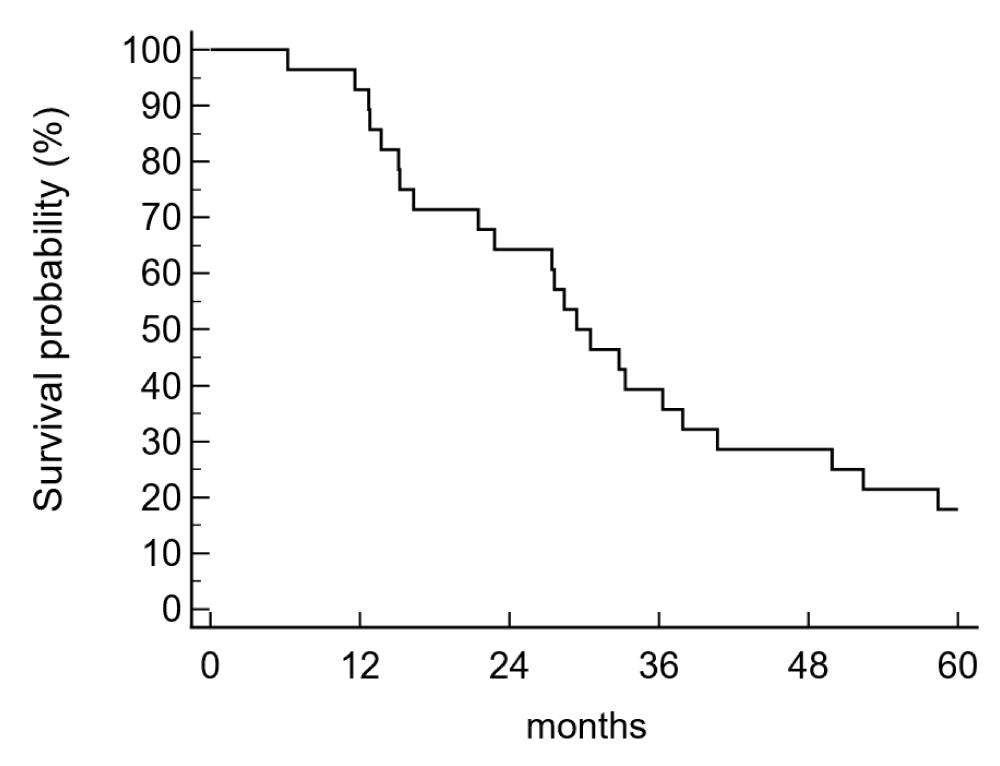
The disease-free survival (DFS) and the overall survival (OS) were 25,0% and 39,3% after 3 years and 14,3% and 17,9% after five years. The median disease-free survival time was 16,9 months, the median survival was 29,4 months (Figure 1a,b).
Figure 1a: Disease-free survival (5 years) in the adjuvant treatment of pancreatic cancer patients (n = 28) was 14,3% and the median disease-free survival time was 16,9 months.
Figure 1b: Overall survival (5 years) in the adjuvant treatment of pancreatic cancer patients (n = 28) was 17,9% and the median survival time was 29,4 months.
In UICC stage I (Ia and Ib) patients the five-year survival rate was 37,5%. The median DFS and median survivals were 18,6 and 33,3 months, respectively. In advanced stages (IIa, IIb, and III) the five-year survival rate was 10%. The median DFS and the median survivals were 11 months and 27,6 months respectively.
In resectable pancreatic cancer median disease-free survival and median survival under standard therapy (5-FU or gemcitabine-based) according to the guidelines are still unsatisfactory. Median survival with adjuvant 5-FU chemotherapy or Gemcitabine chemotherapy range from 15,6 months to 22,8 months, respectively [3,11]. In our cohort analysis, most patients received 5-FU or Gemzitabine as adjuvant chemotherapies. The median disease-free survival of 16,9 months and the median overall survival of 29,4 months point to an advantage in the adjuvant treatment of pancreatic cancer by additional dendritic cell therapy. Furthermore, it has to be noted that in our series no selection of inclusion criteria was made. In the past years, two clinical studies have been conducted investigating the beneficial effects of adjuvant cellular immunotherapy in pancreatic cancer patients. Both trials showed significantly increased median survival times as compared to standard therapy [7,8]. For example, Lepisto, et al. showed an increase in median survival times to 26 months and Nagai, et al. showed a median survival of over 40 months. Nevertheless, in both studies as well as our data, the five-year survival rate remained unsatisfactory.
In this retrospective analysis, we evaluated the clinical results of 28 patients who were treated in the adjuvant situation with dendritic cells following curative surgical resection for pancreatic cancer. The median disease-free survival times and the median survival times were 16,9 months and 29,4 months respectively. These data are consistent with the findings from smaller series by Lepisto, et al. [7] and Nagai, et al. [8] pointing to a potential beneficial effect of dendritic cell therapy in the adjuvant treatment of pancreatic cancer. It must be noted that these promising previous data were collected from a relatively small number of cases and provide initial evidence for a positive effect of adjuvant therapy with dendritic cells in pancreatic cancer. This has to be verified in larger case series.
Conflict of interest
Frank Gansauge is the production manager and joint owner of the laboratory LDG GmbH, where dendritic cells (LANEX-DC®) were produced.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):7-34. doi: 10.3322/caac.21551. Epub 2019 Jan 8. PMID: 30620402.
- Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018 May;267(5):936-945. doi: 10.1097/SLA.0000000000002234. PMID: 28338509.
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013 Oct 9;310(14):1473-81. doi: 10.1001/jama.2013.279201. PMID: 24104372.
- Conroy T, Hammel P, Hebbar M, Abdelghani MB, Wei AC. Canadian cancer trial group and the unicancer-GI-PRODIGE group. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Eng J Med. 2018; 379: 2395-2406.
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012 Mar 22;12(4):265-77. doi: 10.1038/nrc3258. PMID: 22437871; PMCID: PMC3433802.
- Sadeghzadeh M, Bornehdeli S, Mohahammadrezakhani H, Abolghasemi M, Poursaei E, Asadi M, Zafari V, Aghebati-Maleki L, Shanehbandi D. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 2020 Aug 1;254:117580. doi: 10.1016/j.lfs.2020.117580. Epub 2020 Mar 20. PMID: 32205087.
- Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, Finn OJ, Ramanathan RK. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955-964. PMID: 19129927; PMCID: PMC2614325.
- Nagai K, Adachi T, Harada H, Eguchi S, Sugiyama H, Miyazaki Y. Dendritic Cell-based Immunotherapy Pulsed With Wilms Tumor 1 Peptide and Mucin 1 as an Adjuvant Therapy for Pancreatic Ductal Adenocarcinoma After Curative Resection: A Phase I/IIa Clinical Trial. Anticancer Res. 2020 Oct;40(10):5765-5776. doi: 10.21873/anticanres.14593. PMID: 32988904.
- Gansauge F, Poch B, Kleef R, Schwarz M. Effectivity of long antigen exposition dendritic cell therapy (LANEXDC®) in the palliative treatment of pancreatic cancer. Curr Med Chem. 2013;20(38):4827-35. doi: 10.2174/09298673113206660290. PMID: 24083599.
- Gansauge F, Poch B. Effectivity of Long Antigen Exposition Dendritic Cell Therapy (LANEX-DC®) in the Adjuvant Treatment of Gastric Cancer. Clin Oncol. 2022; 7: 1929-31.
- Maeda S, Unno M, Yu J. Adjuvant and neoadjuvant therapy for pancreatic cancer. J Pancreatol. 2019; 2: 100-106.