Abstract
Case Report
Durable Response to Pembrolizumab and Lenvatinib in a Patient with Chemotherapy-refractory Cholangiocarcinoma
Soe P Winn* and Yiwu Huang
Published: 18 July, 2024 | Volume 8 - Issue 1 | Pages: 041-047
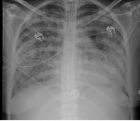
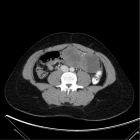
Cholangiocarcinoma (CCA), a rare malignancy originating from bile duct epithelial cells, often presents a challenging prognosis due to its rarity, delayed diagnosis, and early recurrence post-curative-intent treatments. Additional complexities include difficulties in achieving R0 resection during surgical intervention and the lack of effective second-line treatments following the failure of first-line regimens, particularly in unresectable advanced cases.
In this case study, we demonstrate a durable response to a combination regimen of pembrolizumab and lenvatinib in a patient with distal CCA. Despite the regimen’s interim median Progression-Free Survival (PFS) of 6.1 months (95% CI, 2.1-6.4), our patient achieved a clinical and radiological PFS of approximately two years. The underlying mechanisms, potentially involving the upregulation of immune response pathways through undisclosed means or influenced by lenvatinib’s activation of T cells, might augment the sensitivity to PD-1 antibodies like pembrolizumab, contributing to the patient’s sustained response over two years.
This case also highlights the significance of the patient’s initial good health condition, multidisciplinary care, and the potential impact of molecular subtyping on treatment selection in a patient with distal CCA who underwent numerous diagnostic procedures, intricate surgical interventions, and subsequent treatment regimens over seven years. Additionally, we underscore significant landmark trials and emerging combination therapies, including chemotherapies, immunotherapy, and targeted treatments in this report.
Read Full Article HTML DOI: 10.29328/journal.acst.1001043 Cite this Article Read Full Article PDF
Keywords:
Distal cholangiocarcinoma; LEAP-005 trial; Pembrolizumab plus Lenvatinib
References
- Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127-140. Available from: https://doi.org/10.1016/j.annonc.2022.10.506
- Patel N, Benipal B. Incidence of Cholangiocarcinoma in the USA from 2001 to 2015: A US Cancer Statistics Analysis of 50 States. Cureus. 2019;11(1):e3962. Available from: https://doi.org/10.7759/cureus.3962
- Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848-854. Available from: https://pubmed.ncbi.nlm.nih.gov/22173164/
- Zheng S, Zhu Y, Zhao Z, Wu Z, Okanurak K, Lv Z. Liver fluke infection and cholangiocarcinoma: a review. Parasitol Res. 2017;116(1):11-19. Available from: https://pubmed.ncbi.nlm.nih.gov/27718017/
- de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341(18):1368-1378. Available from: https://pubmed.ncbi.nlm.nih.gov/10536130/
- Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13-21. Available from: https://pubmed.ncbi.nlm.nih.gov/22982100/
- Kim BH, Kim K, Chie EK, Kwon J, Jang JY, Kim SW, et al. Long-Term Outcome of Distal Cholangiocarcinoma after Pancreaticoduodenectomy Followed by Adjuvant Chemoradiotherapy: A 15-Year Experience in a Single Institution. Cancer Res Treat. 2017;49(2):473-483. Available from: https://pubmed.ncbi.nlm.nih.gov/27554480/
- Hong SM, Pawlik TM, Cho H, Aggarwal B, Goggins M, Hruban RH, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009;146(2):250-257. Available from: https://pubmed.ncbi.nlm.nih.gov/19628081/
- DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma. Ann Surg. 2007;245(5):755-762. Available from: https://journals.lww.com/annalsofsurgery/abstract/2007/05000/cholangiocarcinoma__thirty_one_year_experience.13.aspx
- Komaya K, Ebata T, Shirai K, Ohira S, Morofuji N, Akutagawa A, et al. Nagoya Surgical Oncology Group. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 2017;104(4):426-433. Available from: https://pubmed.ncbi.nlm.nih.gov/28138968/
- Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97(1):56-64. Available from: https://pubmed.ncbi.nlm.nih.gov/19937985/
- Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver. 2009;3(4):298-305. Available from: https://pubmed.ncbi.nlm.nih.gov/20431764/
- Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469-474. Available from: https://pubmed.ncbi.nlm.nih.gov/20628385/
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. Available from: https://pubmed.ncbi.nlm.nih.gov/20375404/
- Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15(3):478-483. Available from: https://pubmed.ncbi.nlm.nih.gov/14998852/
- Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, et al. EORTC Gastro Intestinal Tract Cancer Group. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41(3):398-403. Available from: https://pubmed.ncbi.nlm.nih.gov/15691639/
- Halim A, Ebrahim MA, Saleh Y. A phase II study of outpatient biweekly gemcitabine-oxaliplatin in advanced biliary tract carcinomas. Jpn J Clin Oncol. 2011;41(2):217-224. Available from: https://pubmed.ncbi.nlm.nih.gov/21062755/
- Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95(7):848-52. Available from: https://pubmed.ncbi.nlm.nih.gov/16969352/
- Jang JS, Lim HY, Hwang IG, Song HS, Yoo N, Yoon S, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol. 2010;65(4):641-7. Available from: https://pubmed.ncbi.nlm.nih.gov/19652971/
- Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, et al. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4(12):1707-1712. Available from: https://pubmed.ncbi.nlm.nih.gov/30178032/
- Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5(6):824-830. Available from: https://pubmed.ncbi.nlm.nih.gov/30998813/
- Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, et al. Kansai Hepatobiliary Oncology Group (KHBO). Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30(1):102-110. Available from: https://pubmed.ncbi.nlm.nih.gov/35900311/
- Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, et al. PRODIGE 38 AMEBICA Investigators/Collaborators. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J Clin Oncol. 2022;40(3):262-271. Available from: https://pubmed.ncbi.nlm.nih.gov/34662180/
- Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012 ;2(3):260-269. Available from: https://pubmed.ncbi.nlm.nih.gov/22585996/
- Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. N Engl J Med Evidence. 2022;1(8). Available from: https://evidence.nejm.org/doi/10.1056/EVIDoa2200015
- Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853-1865. Available from: https://pubmed.ncbi.nlm.nih.gov/37075781/
- Villanueva L, Lwin Z, Chung HC, Gomez-Roca C, Longo F, Yanez E, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J Clin Oncol. 2021 ;39(3_suppl):321-321. Available from: https://ascopubs.org/doi/10.1200/JCO.2021.39.3_suppl.321
- Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019 Feb;14(2). Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0212513
- Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993-4002. Available from: https://pubmed.ncbi.nlm.nih.gov/30447042/
- Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol. 2020 Sep 10;38(26):2981-2992. Available from: https://pubmed.ncbi.nlm.nih.gov/32167863/
- Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Study 309–KEYNOTE-775 Investigators. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022;386(5):437-448. Available from: https://pubmed.ncbi.nlm.nih.gov/35045221/
- Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289-1300. Available from: https://pubmed.ncbi.nlm.nih.gov/33616314/
- Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057-1065. Available from: https://pubmed.ncbi.nlm.nih.gov/32589866/
- Lwin Z, Gomez-Roca C, Saada-Bouzid E, Yanez E, Longo Muñoz F, Im SA, et al. LBA41 LEAP-005: Phase II study of lenvatinib (len) plus pembrolizumab (pembro) in patients (pts) with previously treated advanced solid tumours. Ann Oncol. 2020;31(Suppl 4). Available from: https://www.annalsofoncology.org/article/S0923-7534(20)42353-1/fulltext.
- Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328-2338. Available from: https://pubmed.ncbi.nlm.nih.gov/24769639/
- Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690-701. Available from: https://pubmed.ncbi.nlm.nih.gov/33798493/
- Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22(11):1560-1572. Available from: https://pubmed.ncbi.nlm.nih.gov/34656226/
- Vogel A, Wenzel P, Folprecht G, Schütt P, Wege H, Kretzschmar A, et al. 53MO Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabine-based therapies (NALIRICC – AIO-HEP-0116). Ann Oncol. 2022;33(Suppl). Available from: https://www.annalsofoncology.org/article/S0923-7534(22)01932-9/fulltext
- Demols A, Borbath I, Van den Eynde M, Houbiers G, Peeters M, Marechal R, et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann Oncol. 2020;31(9):1169-1177. Available from: https://pubmed.ncbi.nlm.nih.gov/32464280/
- Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res. 2018 Sep 1;24(17):4154-4161. Available from: https://pubmed.ncbi.nlm.nih.gov/29848569/
- Silverman IM, Murugesan K, Lihou CF, Féliz L, Frampton GM, Newton RC, et al. Comprehensive genomic profiling in FIGHT-202 reveals the landscape of actionable alterations in advanced cholangiocarcinoma. J Clin Oncol. 2019 ;37(15_suppl):4080-4080. Available from: https://ascopubs.org/doi/10.1200/JCO.2019.37.15_suppl.4080
- Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116-1135. Available from: https://pubmed.ncbi.nlm.nih.gov/28667006/
Figures:
Similar Articles
Recently Viewed
-
Clinical and Histopathological Mismatch: A Case Report of Acral FibromyxomaMonica Mishra*,Kailas Mulsange,Gunvanti Rathod,Deepthi Konda. Clinical and Histopathological Mismatch: A Case Report of Acral Fibromyxoma. Arch Pathol Clin Res. 2025: doi: 10.29328/journal.apcr.1001045; 9: 005-007
-
Unconventional powder method is a useful technique to determine the latent fingerprint impressionsHarshita Niranjan,Shweta Rai,Kapil Raikwar,Chanchal Kamle,Rakesh Mia*. Unconventional powder method is a useful technique to determine the latent fingerprint impressions. J Forensic Sci Res. 2022: doi: 10.29328/journal.jfsr.1001035; 6: 045-048
-
Doppler Evaluation of Renal Vessels in Pediatric Patients with Relapse and Remission in Different Categories of Nephrotic SyndromeAmit Nandan Dhar Dwivedi*, Srishti Sharma, OP Mishra, Girish Singh. Doppler Evaluation of Renal Vessels in Pediatric Patients with Relapse and Remission in Different Categories of Nephrotic Syndrome. J Clini Nephrol. 2023: doi: 10.29328/journal.jcn.1001112; 7: 067-072
-
Atlantoaxial subluxation in the pediatric patient: Case series and literature reviewCatherine A Mazzola*,Catherine Christie,Isabel A Snee,Hamail Iqbal. Atlantoaxial subluxation in the pediatric patient: Case series and literature review. J Neurosci Neurol Disord. 2020: doi: 10.29328/journal.jnnd.1001037; 4: 069-074
-
Intelligent Design of Ecological Furniture in Risk Areas based on Artificial SimulationTorres del Salto Rommy Adelfa*, Bryan Alfonso Colorado Pástor*. Intelligent Design of Ecological Furniture in Risk Areas based on Artificial Simulation. Arch Surg Clin Res. 2024: doi: 10.29328/journal.ascr.1001083; 8: 062-068
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."