Abstract
Research Article
Anti COVID-19 immunity developed as assessed in a community-based oncological center
Benjamín Guix*, Teresa Guix, Marco Panichi, Ines Guix, Iván García, Carles Llebaría, Nicolás Achkar, Luis Quinzaños, Hamza Sentisi, Jose Luís Enríquez, Ana Galván, Cristina Pérez-Sánchez, Víctor González and Carmen León
Published: 07 October, 2020 | Volume 4 - Issue 1 | Pages: 038-041
Introduction: Serology (antibody) tests for the SARS-CoV-2 have been proposed as an instrument to inform health authorities about immunization during the COVID-19 pandemic. As there is a significant part of the population that may have some degree of immunity, it is of great interest to communicate the immunization results obtained in the first 500 healthcare workers (HCW), patients and relatives tested in a community-based Oncological Center.
Materials and methods: Between April 9th, 2020 and May 8th, 2020, a group of healthcare workers (HCW), their families, and general public who had had the COVID-19 or had been in close contact with confirmed cases of COVID-19 were screened for IgG SARS-CoV-2 antibodies. The tests were carried out in a rigorous manner, strictly following the guidelines approved by the Spanish Ministry of Health (Ministerio de Sanidad).
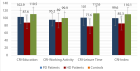
Results: The major objective of this study was to determine the proportion of asymptomatic infected individuals and those who had already secreted IgG against SARS-CoV-2 in our cancer treatment center or in the community of Barcelona. Patients were tested with PCR, Rapid diagnostic test (RDT) or enzyme-linked immunoabsorbent assay (ELISA). A total of 521 participants were tested, 206 with RDT and 315 with ELISA, 59 (11,32%) resulted positive to SARS-CoV-2.
Conclusion: RDT and ELISA proved to be effective and sensible enough to determine the extent of SARS-CoV-2 immunization in a community-based oncological center. The degree of immunization reached is nowadays far away from what can be considered desirable for a herd immunization.
Read Full Article HTML DOI: 10.29328/journal.acst.1001021 Cite this Article Read Full Article PDF
Keywords:
COVID-19; SARS-CoV-2; Seroprevalence; Antibody; PCR; RDT; ELISA; Immunity; Epidemiology; Health care workers; Barcelona; Spain
References
- Gronvall G, Connell N, Kobokobich A, et al. Developing a nacional strategy for serology (antobody testing) in the United States. Johns Hopkins, Bllomberg School of Public Health. Center for health security. www.centerforhealthsecurity.org
- Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020; 25:2000180. PubMed: https://pubmed.ncbi.nlm.nih.gov/32183930/
- Whitehead S, Feibel C. CDC director on models for the months to come: ‘this virus is going to be with us.’ NPR.org March 31, 2020. https://www.npr.org/sections/health-shots/2020/03/31/824155179/cdc-director-on-models-for-the-months-to-come-this-virus-is-going-to-be-with-us
- Interpretación de las pruebas diagnósticas frente a SARS-CoV2. Version 2. Ministerio Sanidad. Instituto de Salud Carlos III. Gobierno de España, 2020.
- Resolución SLT/936/2020 de 4 de mayo, CVE-DOGC-B-20126004-2020.
- Recomendaciones para el manejo, prevención y control de COVID-19 en los ervicios de oncología radioterápica. Ministerio de Sanidad, Sociedad Española de Oncología Radioterápica. 2020.
- Kowk KO, Lai F, Wei W, Wong SYS, Tang JWT. Herdimmunity-estimating the level required to halt COVID-19 epidemies in affected countries. J Infect 2020; 80: e32-e33. PubMed: https://pubmed.ncbi.nlm.nih.gov/32209383/
- Estudio ENE-COVID19: Primera Ronda. Estudio nacional de sero-epidemiología de la infección por SARS-CoV-2 en España. Informe preliminar 13 de mayo de 2020. Ministerio de Ciencia e Innovación, Ministerio de Sanidad, Consejo Interterritorial Sistema Nacional de Salud, Instituto de Salud Carlos III. 2020.
- Streeck H, Schulte B, Kümmerer B, et al. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event. www.ukbonn.de
- Lundkvist, A. Test provide new picture of the spread of the virus. Uppsala universitet. https://www.uu.se/en/news-media/news/article/?id=14756&typ=artikel
- Coronavirus (COVID-19) survey pilor: 2020 https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/28may2020#measuring-the-data
- Garcia-Basteiro A, Moncunill G, Tortajada M, Vidal M, Guinovart C, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 11: 3500. PubMed: https://pubmed.ncbi.nlm.nih.gov/32641730/
- Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, P. Abascal L. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain. J Hosp Infect. 2020; 106: 357–363. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7371579/
Similar Articles
-
Anti COVID-19 immunity developed as assessed in a community-based oncological centerBenjamín Guix*,Teresa Guix,Marco Panichi,Ines Guix,Iván García,Carles Llebaría,Nicolás Achkar,Luis Quinzaños,Hamza Sentisi,Jose Luís Enríquez,Ana Galván,Cristina Pérez-Sánchez,Víctor González,Carmen León. Anti COVID-19 immunity developed as assessed in a community-based oncological center. . 2020 doi: 10.29328/journal.acst.1001021; 4: 038-041
-
COVID-19, Long COVID, and Gastrointestinal Neoplasms: Exploring the Impact of Gut Microbiota and Oncogenic InteractionsAmália Cinthia Meneses do Rêgo,Irami Araújo-Filho*. COVID-19, Long COVID, and Gastrointestinal Neoplasms: Exploring the Impact of Gut Microbiota and Oncogenic Interactions. . 2024 doi: 10.29328/journal.acst.1001045; 8: 054-062
Recently Viewed
-
Toxicity and Phytochemical Analysis of Five Medicinal PlantsJohnson-Ajinwo Okiemute Rosa*, Nyodee, Dummene Godwin. Toxicity and Phytochemical Analysis of Five Medicinal Plants. Arch Pharm Pharma Sci. 2024: doi: 10.29328/journal.apps.1001054; 8: 029-040
-
Antibacterial Screening of Lippia origanoides Essential Oil on Gram-negative BacteriaRodrigo Marcelino Zacarias de Andrade, Bernardina de Paixão Santos, Roberson Matteus Fernandes Silva, Mateus Gonçalves Silva*, Igor de Sousa Oliveira, Sávio Benvindo Ferreira, Rafaelle Cavalcante Lira. Antibacterial Screening of Lippia origanoides Essential Oil on Gram-negative Bacteria. Arch Pharm Pharma Sci. 2024: doi: 10.29328/journal.apps.1001053; 8: 024-028.
-
Next Generation Tools in mRNA Purification: The Role of Continuous Raman Spectroscopy Testing with Pretreatment of the SampleLuisetto M*, Nili B Ahmadabadi, Khaled Edbey, Oleg Yurevich Latyshev. Next Generation Tools in mRNA Purification: The Role of Continuous Raman Spectroscopy Testing with Pretreatment of the Sample. Arch Pharm Pharma Sci. 2024: doi: 10.29328/journal.apps.1001052; 8: 021-023
-
COVID-19 in pregnancy: Our experience at a tertiary maternity unit in FranceDarido Jessie*,El Haddad Cynthia,Diari Jed,Grevoul Fesquet Julie,Bouzid Nassima,Bobric Andrea,Lakhdara Nefissa,Bazzi Zeinab,Lebis Cindy,Khadam Louay,Rigonnot Luc. COVID-19 in pregnancy: Our experience at a tertiary maternity unit in France. Clin J Obstet Gynecol. 2020: doi: 10.29328/journal.cjog.1001051; 3: 054-064
-
Correlation of Inappropriate use of Ceftriaxone and Bacterial Resistance in the Hospital Environment: Integrative ReviewLarissa Furtado Abrantes, Joyce Lima de Sousa, Joel Messias Soares Ramos, Rafael Rodrigues Leite, Sávio Benvindo Ferreira*. Correlation of Inappropriate use of Ceftriaxone and Bacterial Resistance in the Hospital Environment: Integrative Review. Arch Pharm Pharma Sci. 2024: doi: 10.29328/journal.apps.1001051; 8: 014-020
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."